Page 324 - The ROV Manual - A User Guide for Remotely Operated Vehicles 2nd edition
P. 324
316 CHAPTER 12 Sensor Theory
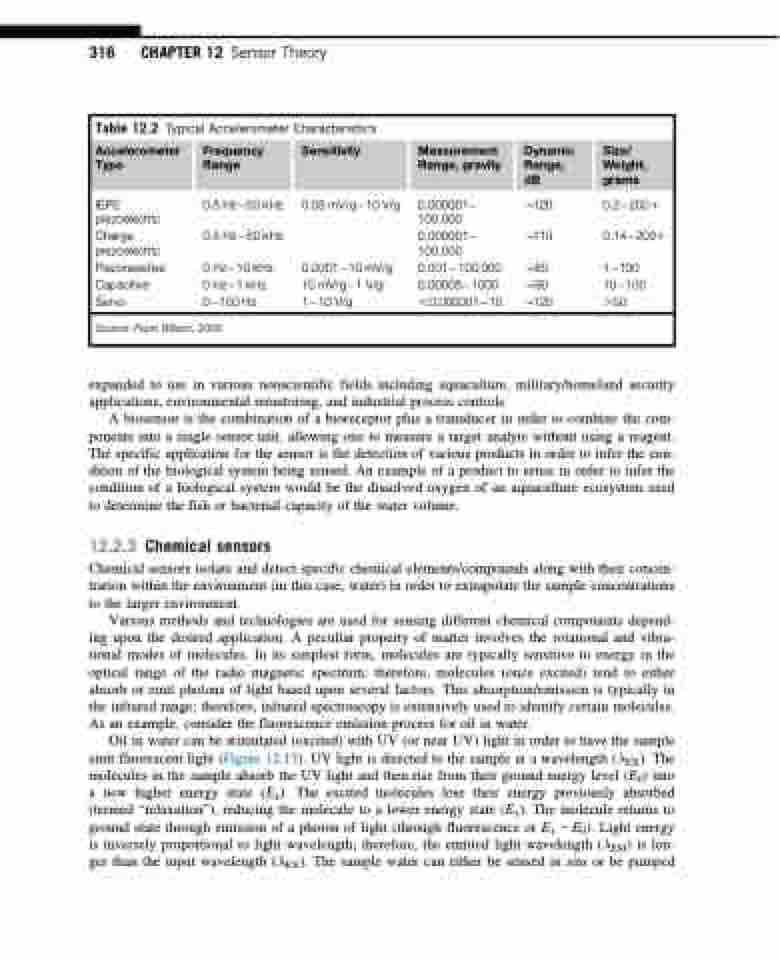
Table 12.2 Typical Accelerometer Characteristics
Accelerometer Type
Frequency Range
Sensitivity
Measurement Range, gravity
Dynamic Range, dB
Size/ Weight, grams
IEPE
piezoelectric
Charge piezoelectric
Piezoresistive Capacitive Servo
0.5 Hz50 kHz 0.5 Hz50 kHz
0 Hz10 kHz 0 Hz1 kHz 0100 Hz
0.05 mV/g10 V/g
0.000110 mV/g 10 mV/g1 V/g 110 V/g
0.000001 B120
100,000
0.000001 B110 100,000
0.001100,000 B80 0.000051000 B90 ,0.00000110 B120
0.22001 0.142001
1100 10100 .50
Source: From Wilson, 2005.
expanded to use in various nonscientific fields including aquaculture, military/homeland security applications, environmental monitoring, and industrial process controls.
A biosensor is the combination of a bioreceptor plus a transducer in order to combine the com- ponents into a single sensor unit, allowing one to measure a target analyte without using a reagent. The specific application for the sensor is the detection of various products in order to infer the con- dition of the biological system being sensed. An example of a product to sense in order to infer the condition of a biological system would be the dissolved oxygen of an aquaculture ecosystem used to determine the fish or bacterial capacity of the water volume.
12.2.3 Chemical sensors
Chemical sensors isolate and detect specific chemical elements/compounds along with their concen- tration within the environment (in this case, water) in order to extrapolate the sample concentrations to the larger environment.
Various methods and technologies are used for sensing different chemical components depend- ing upon the desired application. A peculiar property of matter involves the rotational and vibra- tional modes of molecules. In its simplest form, molecules are typically sensitive to energy in the optical range of the radio magnetic spectrum; therefore, molecules (once excited) tend to either absorb or emit photons of light based upon several factors. This absorption/emission is typically in the infrared range; therefore, infrared spectroscopy is extensively used to identify certain molecules. As an example, consider the fluorescence emission process for oil in water.
Oil in water can be stimulated (excited) with UV (or near UV) light in order to have the sample emit fluorescent light (Figure 12.15). UV light is directed to the sample at a wavelength (λEX). The molecules in the sample absorb the UV light and then rise from their ground energy level (E0) into a new higher energy state (E2). The excited molecules lose their energy previously absorbed (termed “relaxation”), reducing the molecule to a lower energy state (E1). The molecule returns to ground state through emission of a photon of light (through fluorescence or E1 2 E0). Light energy is inversely proportional to light wavelength; therefore, the emitted light wavelength (λEM) is lon- ger than the input wavelength (λEX). The sample water can either be sensed in situ or be pumped