Page 39 - The ROV Manual - A User Guide for Remotely Operated Vehicles 2nd edition
P. 39
Ice at 0C has a density of 57.25 lb/ft3 (0.917 g/cm3), which is about 8% less than that of water at the same temperature. Obviously, water expands when it freezes, bursting pipes and breaking apart water-encrusted rocks, thus producing revenues for marine and land plumbing contractors.
2.2.1.2 Fresh water
A vast majority of the world’s fresh water supply is locked within the ice caps and glaciers of the high Arctic and Antarctic regions. Fresh water is vital to man’s survival, and thus most human endeavors surround areas of fresh water. Due to the shallow water nature of the fresh water collec- tion points, man has placed various items of machinery, structures, and tooling in and around these locations. The ROV pilot will, in all likelihood, have plenty of opportunity to operate within the fresh water environment.
The properties of water directly affect the operation of ROV equipment in the form of tempera- ture (affecting components and electronics), chemistry (affecting seals, incurring oxidation, and degrading machinery operation), and specific gravity (buoyancy and performance). These param- eters will determine the buoyancy of vehicles, the efficiency of thrusters, the numbers and types of biological specimens encountered, as well as the freezing and boiling points of the operational envi- ronment. The water density will further affect sound propagation characteristics, directly impacting the operation of sonar and acoustic positioning equipment.
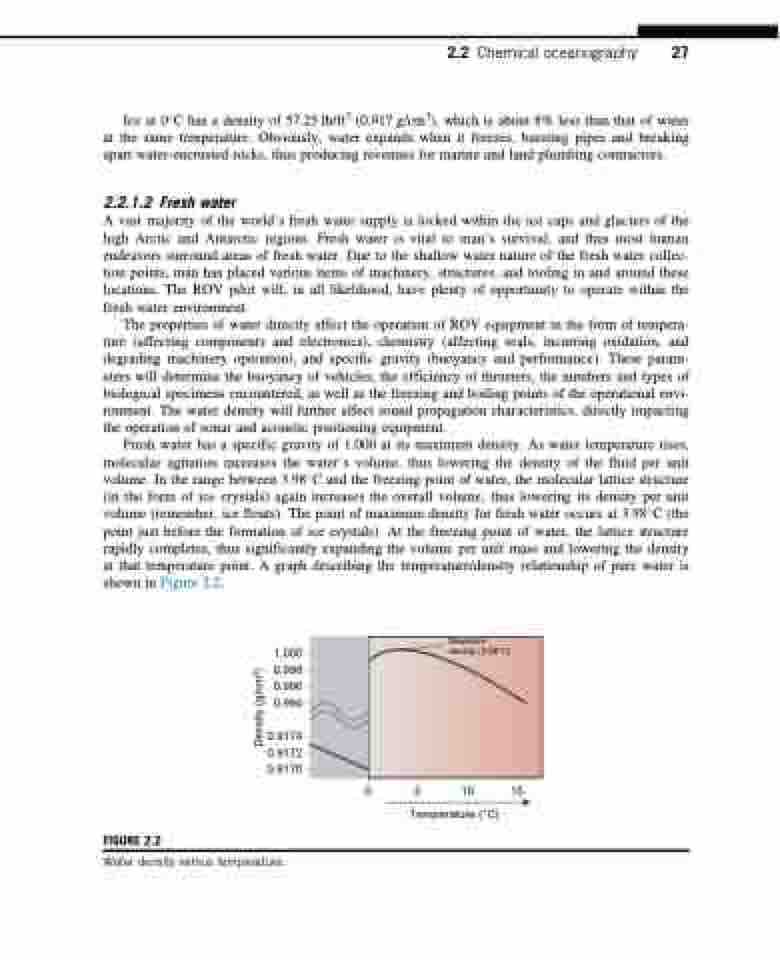
Fresh water has a specific gravity of 1.000 at its maximum density. As water temperature rises, molecular agitation increases the water’s volume, thus lowering the density of the fluid per unit volume. In the range between 3.98C and the freezing point of water, the molecular lattice structure (in the form of ice crystals) again increases the overall volume, thus lowering its density per unit volume (remember, ice floats). The point of maximum density for fresh water occurs at 3.98C (the point just before the formation of ice crystals). At the freezing point of water, the lattice structure rapidly completes, thus significantly expanding the volume per unit mass and lowering the density at that temperature point. A graph describing the temperature/density relationship of pure water is shown in Figure 2.2.
FIGURE 2.2
0 5 10 15
Temperature (°C)
2.2 Chemical oceanography 27
Maximum density (3.98°C)
1.000 0.998 0.996 0.994
0.9174 0.9172 0.9170
Water density versus temperature.
Density (g/cm3)