Page 37 - The ROV Manual - A User Guide for Remotely Operated Vehicles 2nd edition
P. 37
2.2 Chemical oceanography 25
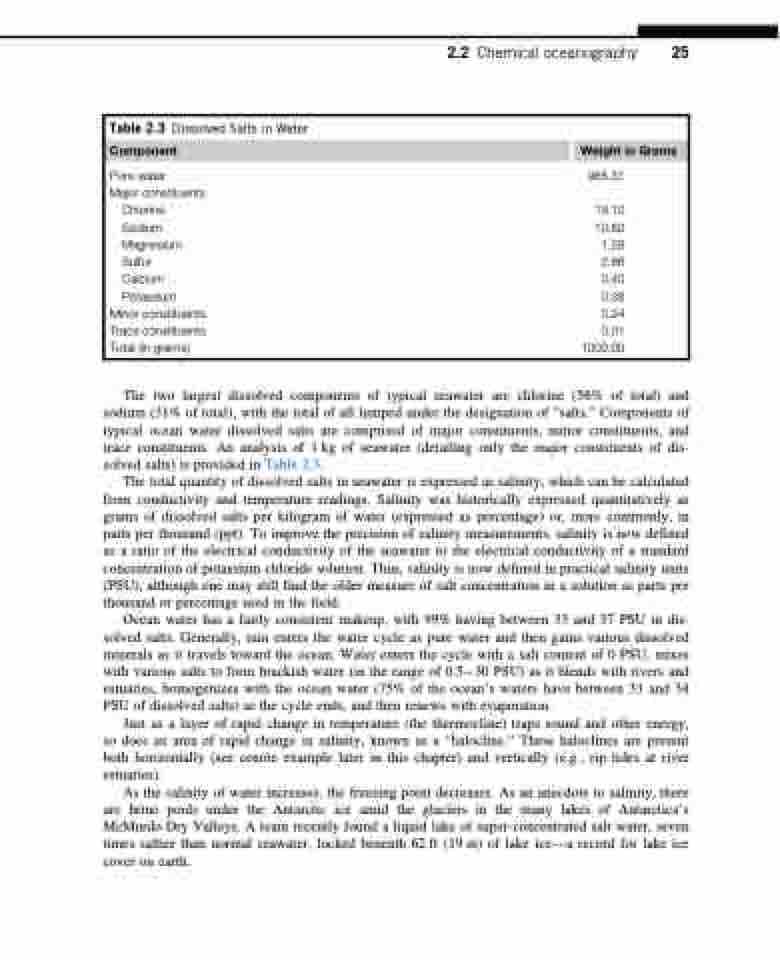
Table 2.3 Dissolved Salts in Water
Component Weight in Grams
Pure water 965.31 Major constituents
Chlorine 19.10 Sodium 10.62 Magnesium 1.28 Sulfur 2.66 Calcium 0.40 Potassium 0.38
Minor constituents 0.24 Trace constituents 0.01 Total (in grams) 1000.00
The two largest dissolved components of typical seawater are chlorine (56% of total) and sodium (31% of total), with the total of all lumped under the designation of “salts.” Components of typical ocean water dissolved salts are comprised of major constituents, minor constituents, and trace constituents. An analysis of 1 kg of seawater (detailing only the major constituents of dis- solved salts) is provided in Table 2.3.
The total quantity of dissolved salts in seawater is expressed as salinity, which can be calculated from conductivity and temperature readings. Salinity was historically expressed quantitatively as grams of dissolved salts per kilogram of water (expressed as percentage) or, more commonly, in parts per thousand (ppt). To improve the precision of salinity measurements, salinity is now defined as a ratio of the electrical conductivity of the seawater to the electrical conductivity of a standard concentration of potassium chloride solution. Thus, salinity is now defined in practical salinity units (PSU), although one may still find the older measure of salt concentration in a solution as parts per thousand or percentage used in the field.
Ocean water has a fairly consistent makeup, with 99% having between 33 and 37 PSU in dis- solved salts. Generally, rain enters the water cycle as pure water and then gains various dissolved minerals as it travels toward the ocean. Water enters the cycle with a salt content of 0 PSU, mixes with various salts to form brackish water (in the range of 0.530 PSU) as it blends with rivers and estuaries, homogenizes with the ocean water (75% of the ocean’s waters have between 33 and 34 PSU of dissolved salts) as the cycle ends, and then renews with evaporation.
Just as a layer of rapid change in temperature (the thermocline) traps sound and other energy, so does an area of rapid change in salinity, known as a “halocline.” These haloclines are present both horizontally (see cenote example later in this chapter) and vertically (e.g., rip tides at river estuaries).
As the salinity of water increases, the freezing point decreases. As an anecdote to salinity, there are brine pools under the Antarctic ice amid the glaciers in the many lakes of Antarctica’s McMurdo Dry Valleys. A team recently found a liquid lake of super-concentrated salt water, seven times saltier than normal seawater, locked beneath 62 ft (19 m) of lake ice—a record for lake ice cover on earth.