Page 51 - The ROV Manual - A User Guide for Remotely Operated Vehicles 2nd edition
P. 51
1
2
3 3.5 4
5 5.5 6
7 8
9
10 11 12
13 14
Hydrochloric acid
Lime juice
Acetic acid Acid rain Tomato juice
Black coffee River water Milk
Pure water; blood Seawater; baking soda
Borax solution
Household ammonia
Sodium hydroxide
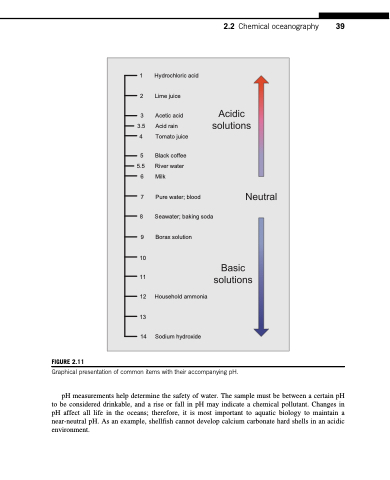
FIGURE 2.11
Graphical presentation of common items with their accompanying pH.
pH measurements help determine the safety of water. The sample must be between a certain pH to be considered drinkable, and a rise or fall in pH may indicate a chemical pollutant. Changes in pH affect all life in the oceans; therefore, it is most important to aquatic biology to maintain a near-neutral pH. As an example, shellfish cannot develop calcium carbonate hard shells in an acidic environment.
2.2 Chemical oceanography 39