Page 53 - The ROV Manual - A User Guide for Remotely Operated Vehicles 2nd edition
P. 53
2.2 Chemical oceanography 41
Table 2.4 Distribution of Gases in the Atmosphere and Dissolved in Seawater
Gas
Nitrogen (N2)
Oxygen (O2)
Carbon dioxide (CO2)
78 48 11 21 36 6 0.04 15 83
Percentage of Gas Phase by Volume
Atmosphere Surface Oceans Total Oceans
Source: Segar (1998).
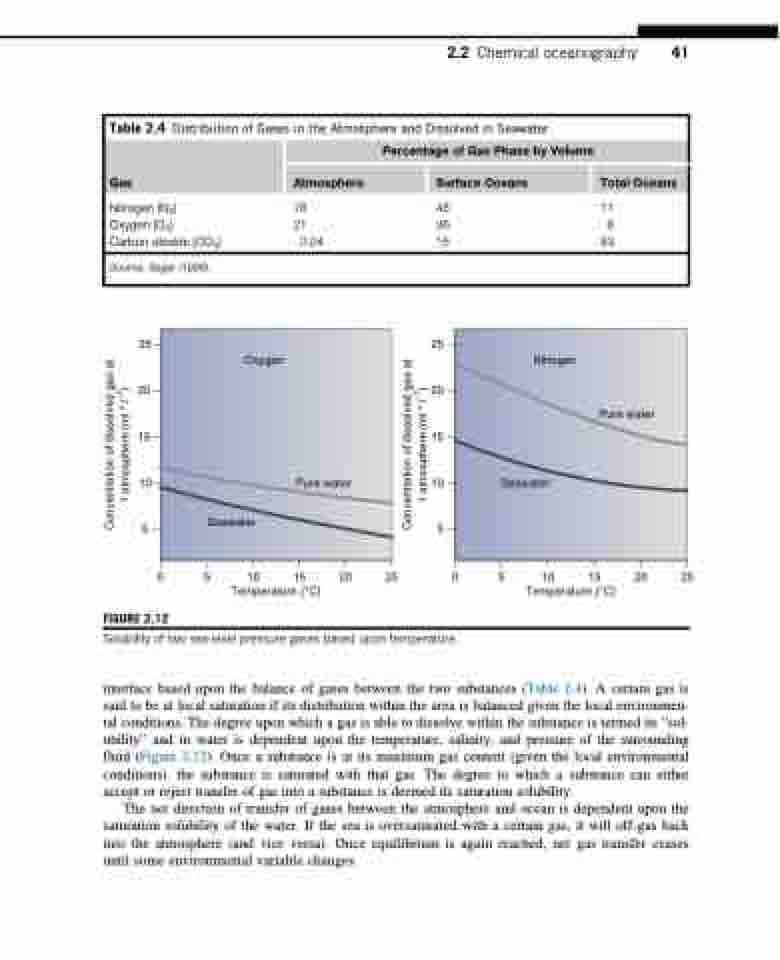
25 25
20 20
15 15
10 10
55
0 5 10 15 20 25 Temperature (°C)
FIGURE 2.12
0 5 10 15 20 25 Temperature (°C)
Seawater
Oxygen
Nitrogen
Seawater
Pure water
Pure water
Solubility of two sea-level pressure gases based upon temperature.
interface based upon the balance of gases between the two substances (Table 2.4). A certain gas is said to be at local saturation if its distribution within the area is balanced given the local environmen- tal conditions. The degree upon which a gas is able to dissolve within the substance is termed its “sol- ubility” and in water is dependent upon the temperature, salinity, and pressure of the surrounding fluid (Figure 2.12). Once a substance is at its maximum gas content (given the local environmental conditions), the substance is saturated with that gas. The degree to which a substance can either accept or reject transfer of gas into a substance is deemed its saturation solubility.
The net direction of transfer of gases between the atmosphere and ocean is dependent upon the saturation solubility of the water. If the sea is oversaturated with a certain gas, it will off-gas back into the atmosphere (and vice versa). Once equilibrium is again reached, net gas transfer ceases until some environmental variable changes.
Concentration of dissolved gas at 1 atmosphere (ml * l–1)
Concentration of dissolved gas at 1 atmosphere (ml * l–1)