Page 668 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 668
654 SECTION VI Drugs Used to Treat Diseases of the Blood, Inflammation, & Gout
Adalimumab Infliximab Etanercept
V H V H Extracellular domain
V L V L of human p75 receptor
C H1 C H1
C L C L
C H2 C H2 C H2
F region of F region of
C
C
human IgG 1 human IgG 1
C H3 C H3 C H3
Golimumab Certolizumab
V H
V L
C H1 Humanized Fab
C L fragment
C H2 Polyethylene
glycol (PEG)
F region of
C
human IgG 1
C H3
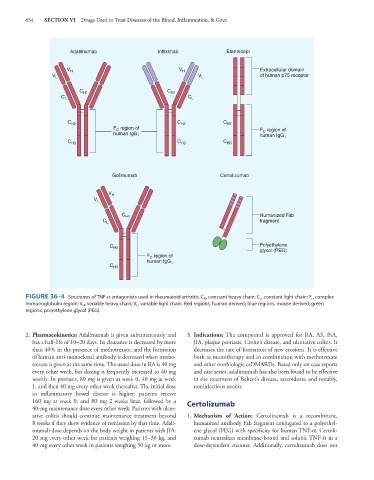
FIGURE 36–4 Structures of TNF-α antagonists used in rheumatoid arthritis. C H , constant heavy chain; C L , constant light chain; F c , complex
immunoglobulin region; V H , variable heavy chain; V L , variable light chain. Red regions, human derived; blue regions, mouse derived; green
regions, polyethylene glycol (PEG).
2. Pharmacokinetics: Adalimumab is given subcutaneously and 3. Indications: The compound is approved for RA, AS, PsA,
has a half-life of 10–20 days. Its clearance is decreased by more JIA, plaque psoriasis, Crohn’s disease, and ulcerative colitis. It
than 40% in the presence of methotrexate, and the formation decreases the rate of formation of new erosions. It is effective
of human anti-monoclonal antibody is decreased when metho- both as monotherapy and in combination with methotrexate
trexate is given at the same time. The usual dose in RA is 40 mg and other nonbiologic csDMARDs. Based only on case reports
every other week, but dosing is frequently increased to 40 mg and case series, adalimumab has also been found to be effective
weekly. In psoriasis, 80 mg is given at week 0, 40 mg at week in the treatment of Behçet’s disease, sarcoidosis, and notably,
1, and then 40 mg every other week thereafter. The initial dose noninfectious uveitis.
in inflammatory bowel disease is higher; patients receive
160 mg at week 0, and 80 mg 2 weeks later, followed by a Certolizumab
40-mg maintenance dose every other week. Patients with ulcer-
ative colitis should continue maintenance treatment beyond 1. Mechanism of Action: Certolizumab is a recombinant,
8 weeks if they show evidence of remission by that time. Adali- humanized antibody Fab fragment conjugated to a polyethyl-
mumab dose depends on the body weight in patients with JIA: ene glycol (PEG) with specificity for human TNF-α. Certoli-
20 mg every other week for patients weighing 15–30 kg, and zumab neutralizes membrane-bound and soluble TNF-α in a
40 mg every other week in patients weighing 30 kg or more. dose-dependent manner. Additionally, certolizumab does not