Page 20 - Policy Wording - Commercial Underwriting Mandates & Guidelines Binder Addendum
P. 20
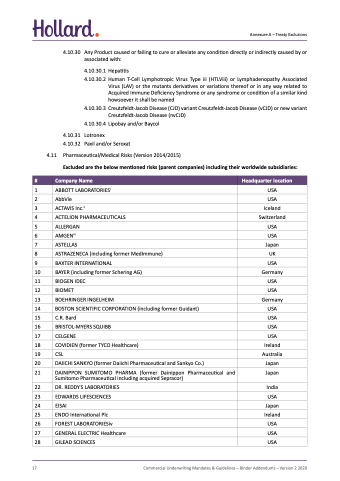
4.10.30 Any Product caused or failing to cure or alleviate any condition directly or indirectly caused by or associated with:
4.10.30.1 Hepatitis
4.10.30.2 Human T-Cell Lymphotropic Virus Type iii (HTLViii) or Lymphadenopathy Associated Virus (LAV) or the mutants derivatives or variations thereof or in any way related to Acquired Immune Deficiency Syndrome or any syndrome or condition of a similar kind howsoever it shall be named
4.10.30.3 Creutzfeldt-Jacob Disease (CJD) variant Creutzfeldt-Jacob Disease (vCJD) or new variant Creutzfeldt-Jacob Disease (nvCJD)
4.10.30.4 Lipobay and/or Baycol
4.10.31 Lotronex
4.10.32 Paxil and/or Seroxat
4.11 Pharmaceutical/Medical Risks (Version 2014/2015)
Excluded are the below mentioned risks (parent companies) including their worldwide subsidiaries:
1 ABBOTT LABORATORIESi USA
2 AbbVie USA
3 ACTAVIS Inc.ii Iceland
4 ACTELION PHARMACEUTICALS Switzerland
5 ALLERGAN USA
6 AMGENiii USA
7 ASTELLAS Japan
8 ASTRAZENECA (including former MedImmune) UK
9 BAXTER INTERNATIONAL USA
10 BAYER (including former Schering AG)
11 BIOGEN IDEC USA
12 BIOMET USA
13 BOEHRINGER INGELHEIM Germany
14 BOSTON SCIENTIFIC CORPORATION (including former Guidant) USA
15 C.R. Bard USA
16 BRISTOL-MYERS SQUIBB USA
17 CELGENE USA
18 COVIDIEN (former TYCO Healthcare) Ireland
19 CSL Australia
Annexure A – Treaty Exclusions
#
Company Name
Headquarter location
Germany
Japan Japan
22 DR. REDDY'S LABORATORIES
23 EDWARDS LIFESCIENCES
24 EISAI Japan
20 DAIICHI SANKYO (former Daiichi Pharmaceutical and Sankyo Co.)
21 DAINIPPON SUMITOMO PHARMA (former Dainippon Pharmaceutical and Sumitomo Pharmaceutical including acquired Sepracor)
India USA
25 ENDO International Plc
26 FOREST LABORATORIESiv
27 GENERAL ELECTRIC Healthcare
28 GILEAD SCIENCES
Ireland USA USA USA
17
Commercial Underwriting Mandates & Guidelines – Binder Addendums – Version 2 2020