Page 4 - E-Science Prep1 2024-2025 t2 Unit1_Neat - Copy
P. 4
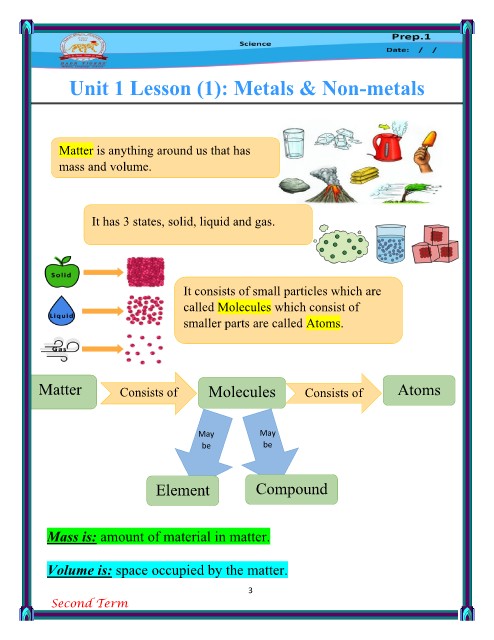
Molecule: The smallest part of matter can exist freely and carry properties of
matter. (2/more atoms join chemically)
Element: The simplest form of the matter that can’t be analyzed.
(Pure substance)
It consists of 1 / more similar atom(s). (1 Type of atoms)
Compound: The combination of atoms of 2/more different elements with
constant weight ratio. (2/more diff. elements join together.)
Atom: The building unit of Ion: The atom of an element
the structure of any matter. loses or gains an electron or more
during the chemical reaction.
Elements
Metals Non- Noble gas
metals
Second Term
4