Page 5 - E-Science Prep1 2024-2025 t2 Unit1_Neat - Copy
P. 5
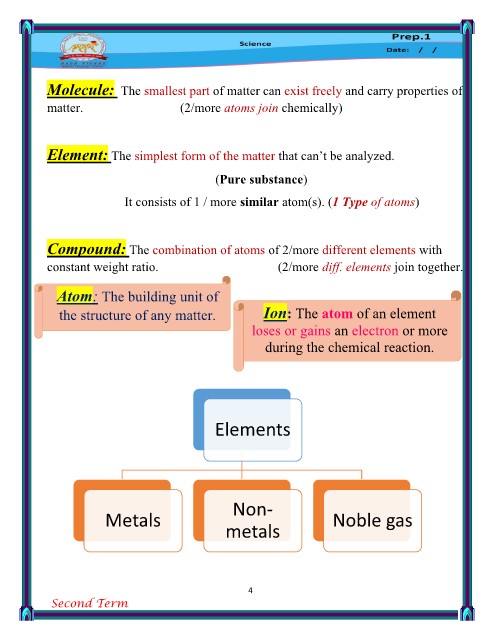
P.O.C Metals Non-metals
1- Definition: They are elements have They are elements have 5, 6
(electrons in the 1, 2 or 3 electrons in the or 7 electrons in the
outermost energy outermost energy level. outermost energy level.
level) Except H1 has 1 & C6 has 4
They are solids except They are solids and gases
2- The state at room mercury (Hg) which is except bromine (Br) which
temperature: the only liquid metal. is the only liquid non-metal.
They have metallic They don’t have metallic
3- Luster (shining): luster (shiny) luster. (opaque)
3- Conductivity of They are good They are bad conductors of
heat and electricity except
heat and conductors of heat and graphite (carbon) which is
used in dry cells.
electricity: electricity. They open the circuit.
They aren’t malleable or
5- Malleability and They close the circuit ductile. (brittle)
ductility: They crumble while
They are malleable and hammering
ductile. Their atoms tend to gain an
electron or more and change
6- During the Their atoms tend to give into negative ions.
chemical reaction: or lose an electron or
more and change into Low
7- Melting point positive ions.
High
8- Examples Sodium, copper, zinc, Carbon, sulphur,
silver & iron phosphorus & iodine.
Copper can be easily shaped Carbon is used in dry cell as it
as it is metal & malleable. 5 is good conductor of electricity
Second Term