Page 100 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 100
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
13
❖ C-NMR resonance are not easy
to observe, due to the natural
abundance of 13 C is very low
(1.1%). (the most abundant isotope
12
is C)
❖ Up to this point and using ordinary
instrument, the only useful
parameter obtained from 13 C is
the chemical shifts (scale: 0-200
ppm) that help in predicting the
No. & type of carbon atoms in a
given sample.
13
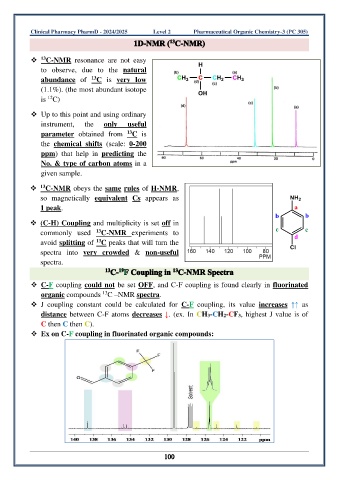
❖ C-NMR obeys the same rules of H-NMR,
so magnetically equivalent Cs appears as
1 peak.
❖ (C-H) Coupling and multiplicity is set off in
commonly used 13 C-NMR experiments to
13
avoid splitting of C peaks that will turn the
spectra into very crowded & non-useful
spectra.
❖ C-F coupling could not be set OFF, and C-F coupling is found clearly in fluorinated
13
organic compounds C –NMR spectra.
❖ J coupling constant could be calculated for C-F coupling, its value increases ↑↑ as
distance between C-F atoms decreases ↓. (ex. In CH3-CH2-CF3, highest J value is of
C then C then C).
❖ Ex on C-F coupling in fluorinated organic compounds: