Page 96 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 96
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
❖ As mentioned, nuclei which spin generate a magnetic field also its electrons spins
producing another magnetic field; this electron clouds magnetic field interaction is
responsible for splitting and coupling constants (J).
J
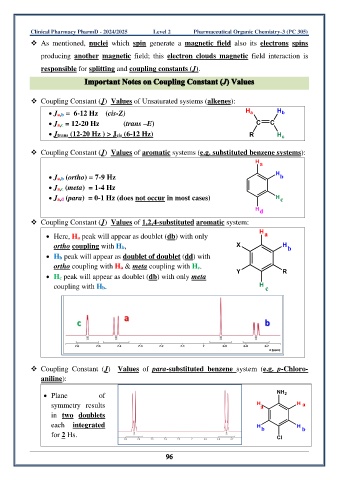
❖ Coupling Constant (J) Values of Unsaturated systems (alkenes):
• Ja,b = 6-12 Hz (cis-Z)
• Ja,c = 12-20 Hz (trans –E)
• Jtrans (12-20 Hz ) > Jcis (6-12 Hz)
❖ Coupling Constant (J) Values of aromatic systems (e.g. substituted benzene systems):
• Ja,b (ortho) = 7-9 Hz
• Ja,c (meta) = 1-4 Hz
• Ja,d (para) = 0-1 Hz (does not occur in most cases)
❖ Coupling Constant (J) Values of 1,2,4-substituted aromatic system:
• Here, Ha peak will appear as doublet (db) with only
ortho coupling with Hb,
• Hb peak will appear as doublet of doublet (dd) with
ortho coupling with Ha & meta coupling with Hc.
• Hc peak will appear as doublet (db) with only meta
coupling with Hb.
❖ Coupling Constant (J) Values of para-substituted benzene system (e.g. p-Chloro-
aniline):
• Plane of
symmetry results
in two doublets
each integrated
for 2 Hs.