Page 92 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 92
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
2
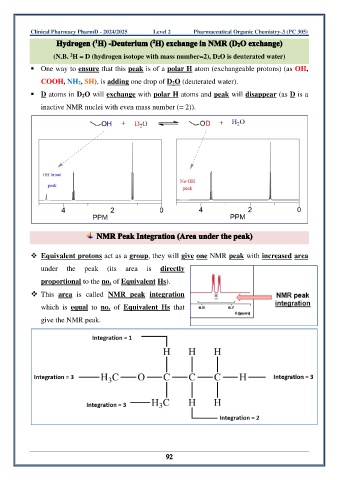
(N.B. H = D (hydrogen isotope with mass number=2), D2O is deuterated water)
▪ One way to ensure that this peak is of a polar H atom (exchangeable protons) (as OH,
COOH, NH2, SH), is adding one drop of D2O (deuterated water).
▪ D atoms in D2O will exchange with polar H atoms and peak will disappear (as D is a
inactive NMR nuclei with even mass number (= 2)).
❖ Equivalent protons act as a group, they will give one NMR peak with increased area
under the peak (its area is directly
proportional to the no. of Equivalent Hs).
❖ This area is called NMR peak integration
which is equal to no. of Equivalent Hs that
give the NMR peak.