Page 90 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 90
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
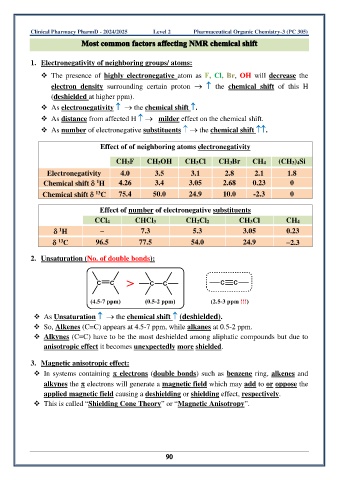
1. Electronegativity of neighboring groups/ atoms:
❖ The presence of highly electronegative atom as F, Cl, Br, OH will decrease the
electron density surrounding certain proton → the chemical shift of this H
(deshielded at higher ppm).
❖ As electronegativity → the chemical shift .
❖ As distance from affected H → milder effect on the chemical shift.
❖ As number of electronegative substituents → the chemical shift .
Effect of of neighboring atoms electronegativity
CH3F CH3OH CH3Cl CH3Br CH4 (CH3)4Si
Electronegativity 4.0 3.5 3.1 2.8 2.1 1.8
1
Chemical shift H 4.26 3.4 3.05 2.68 0.23 0
13
Chemical shift C 75.4 50.0 24.9 10.0 -2.3 0
Effect of number of electronegative substituents
CCl4 CHCl3 CH2Cl2 CH3Cl CH4
1
H – 7.3 5.3 3.05 0.23
13
C 96.5 77.5 54.0 24.9 −2.3
2. Unsaturation (No. of double bonds):
❖ As Unsaturation → the chemical shift (deshielded).
❖ So, Alkenes (C=C) appears at 4.5-7 ppm, while alkanes at 0.5-2 ppm.
❖ Alkynes (C≡C) have to be the most deshielded among aliphatic compounds but due to
anisotropic effect it becomes unexpectedly more shielded.
3. Magnetic anisotropic effect:
❖ In systems containing electrons (double bonds) such as benzene ring, alkenes and
alkynes the electrons will generate a magnetic field which may add to or oppose the
applied magnetic field causing a deshielding or shielding effect, respectively.
❖ This is called “Shielding Cone Theory” or “Magnetic Anisotropy”.