Page 11 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 11
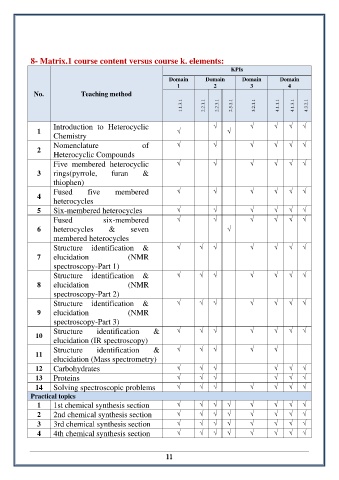
8- Matrix.1 course content versus course k. elements:
KPIs
Domain Domain Domain Domain
1 2 3 4
No. Teaching method
1.1.3.1 2.2.1.1 2.2.3.1 2.5.3.1 3.2.1.1 4.1.1.1 4.1.3.1 4.3.2.1
Introduction to Heterocyclic √ √ √ √ √
1 √ √
Chemistry
Nomenclature of √ √ √ √ √ √
2
Heterocyclic Compounds
Five membered heterocyclic √ √ √ √ √ √
3 rings(pyrrole, furan &
thiophen)
Fused five membered √ √ √ √ √ √
4
heterocycles
5 Six-membered heterocycles √ √ √ √ √ √
Fused six-membered √ √ √ √ √ √
6 heterocycles & seven √
membered heterocycles
Structure identification & √ √ √ √ √ √ √
7 elucidation (NMR
spectroscopy-Part 1)
Structure identification & √ √ √ √ √ √ √
8 elucidation (NMR
spectroscopy-Part 2)
Structure identification & √ √ √ √ √ √ √
9 elucidation (NMR
spectroscopy-Part 3)
Structure identification & √ √ √ √ √ √ √
10
elucidation (IR spectroscopy)
Structure identification & √ √ √ √ √
11
elucidation (Mass spectrometry)
12 Carbohydrates √ √ √ √ √ √
13 Proteins √ √ √ √ √ √
14 Solving spectroscopic problems √ √ √ √ √ √ √
Practical topics
1 1st chemical synthesis section √ √ √ √ √ √ √ √
2 2nd chemical synthesis section √ √ √ √ √ √ √ √
3 3rd chemical synthesis section √ √ √ √ √ √ √ √
4 4th chemical synthesis section √ √ √ √ √ √ √ √