Page 53 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 53
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
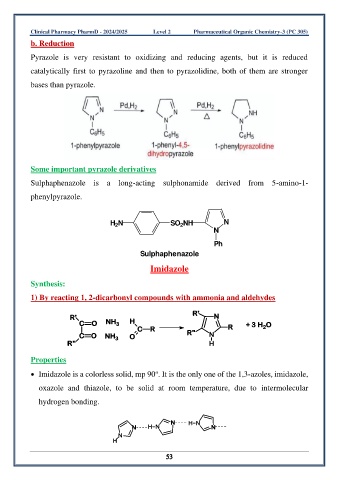
b. Reduction
Pyrazole is very resistant to oxidizing and reducing agents, but it is reduced
catalytically first to pyrazoline and then to pyrazolidine, both of them are stronger
bases than pyrazole.
Some important pyrazole derivatives
Sulphaphenazole is a long-acting sulphonamide derived from 5-amino-1-
phenylpyrazole.
H N SO NH N
2
2
N
Ph
Sulphaphenazole
Imidazole
Synthesis:
1) By reacting 1, 2-dicarbonyl compounds with ammonia and aldehydes
R'
R' N
C O NH 3 H + 3 H O
C R R 2
C O NH 3 O R" N
R" H
Properties
• Imidazole is a colorless solid, mp 90 . It is the only one of the 1,3-azoles, imidazole,
o
oxazole and thiazole, to be solid at room temperature, due to intermolecular
hydrogen bonding.
N H N
N H N N
N
H