Page 30 - Mass General Brigham - Innovators Guide 1.0
P. 30
30 INNO V AT O RS GUIDE | M ASS GENERAL BRIGH AM
Enabling collaboration
3 1 / 3
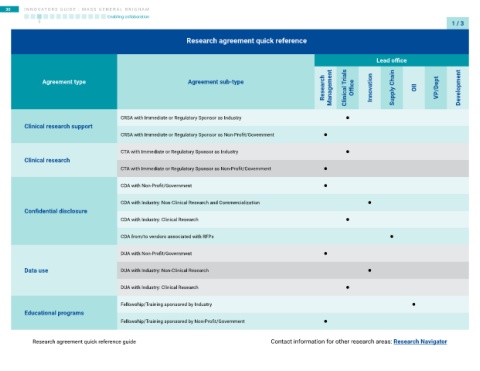
Research agreement quick reference
Lead office
Research Management Clinical Trials Office Innovation Supply Chain OII VP/Dept Development
Agreement type Agreement sub-type
•
CRSA with Immediate or Regulatory Sponsor as Industry
Clinical research support
CRSA with Immediate or Regulatory Sponsor as Non-Profit/Government •
•
CTA with Immediate or Regulatory Sponsor as Industry
Clinical research
CTA with Immediate or Regulatory Sponsor as Non-Profit/Government •
•
CDA with Non-Profit/Government
•
CDA with Industry: Non-Clinical Research and Commercialization
Confidential disclosure
CDA with Industry: Clinical Research •
•
CDA from/to vendors associated with RFPs
•
DUA with Non-Profit/Government
Data use DUA with Industry: Non-Clinical Research •
•
DUA with Industry: Clinical Research
•
Fellowship/Training sponsored by Industry
Educational programs
Fellowship/Training sponsored by Non-Profit/Government •
Research agreement quick reference guide Contact information for other research areas: Research Navigator