Page 22 - IBRO_RNA School_Abstract Book
P. 22
To understand the splicing mechanism in HIV genome
Niyati Jain
CSIR-Institute of Genomics and Integrative Biology, Delhi,
India
immunode v t 1 (HIV i
factor as it regulates the expression of viral proteins. The relative
distribution of particular mRNA transcript that encodes for distinct HIV-1 protein is
determined by the recurrence of splicing at various suboptimal alternative 3’
splice sites (3’ss). Several cis-acting elements have been identified within the viral
genome, those in conjunction with RNA-binding proteins either repress or facili-
tate the splicing event. Heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1)
is an important RNA-binding protein required for controlling HIV genome splicing.
To clarify the mechanism by which the splicing is regulated in HIV, the higher
order architecture need to be determined. Further the determinants of sequence
that define the specific interactions with hnRNPA1 also need to be characterized.
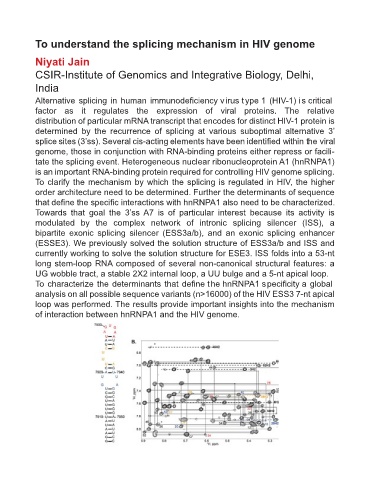
T 3’ is
modulated by the complex network of intronic splicing silencer (ISS), a
bipartite exonic splicing silencer (ESS3a/b), and an exonic splicing enhancer
(ESSE3). We previously solved the solution structure of ESS3a/b and ISS and
currently working to solve the solution structure for ESE3. ISS folds into a 53-nt
long stem-loop RNA composed of several non-canonical structural features: a
UG wobble tract, a stable 2X2 internal loop, a UU bulge and a 5-nt apical loop.
T t hnRNP s global
analysis on all possible sequence variants (n>16000) of the HIV ESS3 7-nt apical
loop was performed. The results provide important insights into the mechanism
of interaction between hnRNPA1 and the HIV genome.