Page 4 - GMP for warehouse
P. 4
GMP Training – GMP for Warehouse by www.gmpsop.com
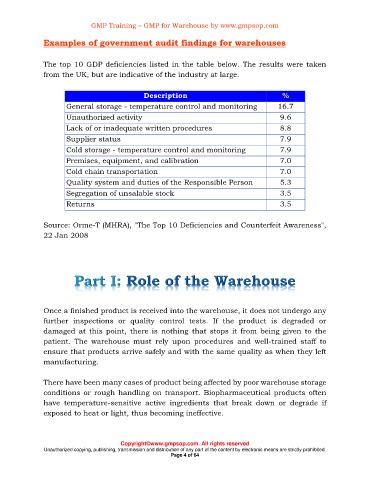
Examples of government audit findings for warehouses
The top 10 GDP deficiencies listed in the table below. The results were taken
from the UK, but are indicative of the industry at large.
Description %
General storage - temperature control and monitoring 16.7
Unauthorized activity 9.6
Lack of or inadequate written procedures 8.8
Supplier status 7.9
Cold storage - temperature control and monitoring 7.9
Premises, equipment, and calibration 7.0
Cold chain transportation 7.0
Quality system and duties of the Responsible Person 5.3
Segregation of unsalable stock 3.5
Returns 3.5
Source: Orme-T (MHRA), "The Top 10 Deficiencies and Counterfeit Awareness",
22 Jan 2008
Once a finished product is received into the warehouse, it does not undergo any
further inspections or quality control tests. If the product is degraded or
damaged at this point, there is nothing that stops it from being given to the
patient. The warehouse must rely upon procedures and well-trained staff to
ensure that products arrive safely and with the same quality as when they left
manufacturing.
There have been many cases of product being affected by poor warehouse storage
conditions or rough handling on transport. Biopharmaceutical products often
have temperature-sensitive active ingredients that break down or degrade if
exposed to heat or light, thus becoming ineffective.
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by electronic means are strictly prohibited.
Page 4 of 64