Page 34 - QA and QC
P. 34
GMP Training – Quality Assurance and Quality Control by www.gmpsop.com
GMP and process control
Under GMP, all manufacturing process steps must be "under control". After
processes are initially validated, to maintain control:
Inputs (CPPs or critical processing parameters) should be monitored
throughout the process.
Outputs (CQAs or critical quality attributes) should also be monitored against
tolerances.
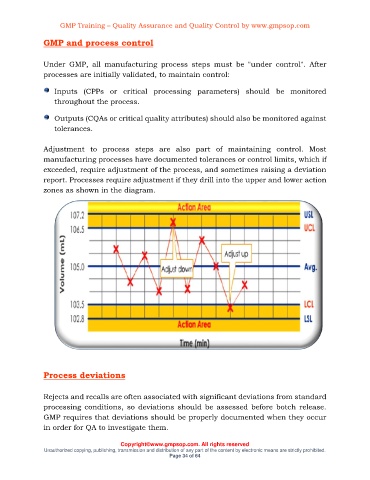
Adjustment to process steps are also part of maintaining control. Most
manufacturing processes have documented tolerances or control limits, which if
exceeded, require adjustment of the process, and sometimes raising a deviation
report. Processes require adjustment if they drill into the upper and lower action
zones as shown in the diagram.
Process deviations
Rejects and recalls are often associated with significant deviations from standard
processing conditions, so deviations should be assessed before botch release.
GMP requires that deviations should be properly documented when they occur
in order for QA to investigate them.
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by electronic means are strictly prohibited.
Page 34 of 64