Page 57 - QA and QC
P. 57
GMP Training – Quality Assurance and Quality Control by www.gmpsop.com
MANAGEMENT RESPONSIBILITY
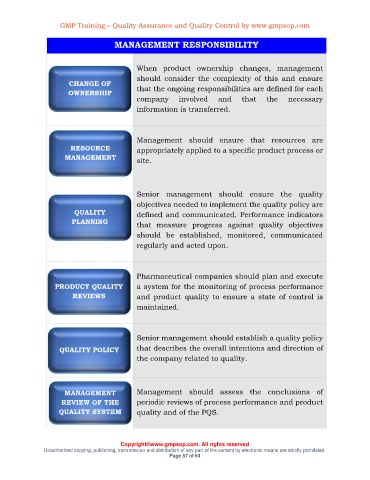
When product ownership changes, management
should consider the complexity of this and ensure
CHANGE OF
OWNERSHIP that the ongoing responsibilities are defined for each
company involved and that the necessary
information is transferred.
Management should ensure that resources are
RESOURCE appropriately applied to a specific product process or
MANAGEMENT site.
Senior management should ensure the quality
objectives needed to implement the quality policy are
QUALITY defined and communicated. Performance indicators
PLANNING that measure progress against quality objectives
should be established, monitored, communicated
regularly and acted upon.
Pharmaceutical companies should plan and execute
PRODUCT QUALITY a system for the monitoring of process performance
REVIEWS and product quality to ensure a state of control is
maintained.
Senior management should establish a quality policy
QUALITY POLICY that describes the overall intentions and direction of
the company related to quality.
MANAGEMENT Management should assess the conclusions of
REVIEW OF THE periodic reviews of process performance and product
QUALITY SYSTEM quality and of the PQS.
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by electronic means are strictly prohibited.
Page 57 of 64