Page 61 - QA and QC
P. 61
GMP Training – Quality Assurance and Quality Control by www.gmpsop.com
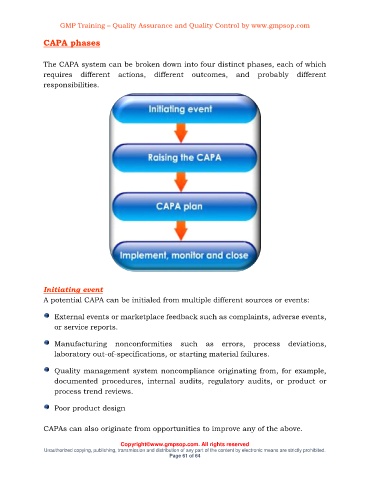
CAPA phases
The CAPA system can be broken down into four distinct phases, each of which
requires different actions, different outcomes, and probably different
responsibilities.
Initiating event
A potential CAPA can be initialed from multiple different sources or events:
External events or marketplace feedback such as complaints, adverse events,
or service reports.
Manufacturing nonconformities such as errors, process deviations,
laboratory out-of-specifications, or starting material failures.
Quality management system noncompliance originating from, for example,
documented procedures, internal audits, regulatory audits, or product or
process trend reviews.
Poor product design
CAPAs can also originate from opportunities to improve any of the above.
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by electronic means are strictly prohibited.
Page 61 of 64