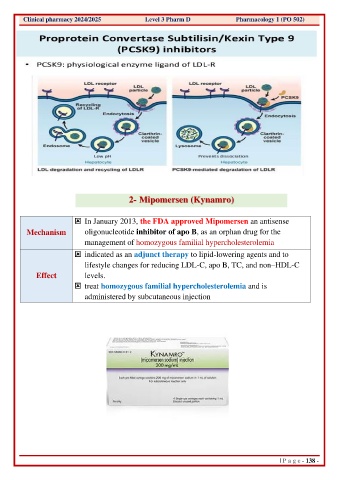
Page 154 - pharma 1 theoretical updated MNU_Neat
P. 154
Clinical pharmacy 2024/2025 Level 3 Pharm D Pharmacology 1 (PO 502)
2- Mipomersen (Kynamro)
In January 2013, the FDA approved Mipomersen an antisense
Mechanism oligonucleotide inhibitor of apo B, as an orphan drug for the
management of homozygous familial hypercholesterolemia
indicated as an adjunct therapy to lipid-lowering agents and to
lifestyle changes for reducing LDL-C, apo B, TC, and non–HDL-C
Effect levels.
treat homozygous familial hypercholesterolemia and is
administered by subcutaneous injection
| P a g e - 138 -