Page 128 - phytochemistry general program
P. 128
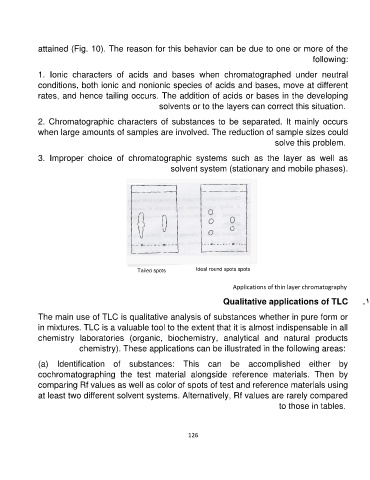
attained (Fig. 10). The reason for this behavior can be due to one or more of the
following:
1. Ionic characters of acids and bases when chromatographed under neutral
conditions, both ionic and nonionic species of acids and bases, move at different
rates, and hence tailing occurs. The addition of acids or bases in the developing
solvents or to the layers can correct this situation.
2. Chromatographic characters of substances to be separated. It mainly occurs
when large amounts of samples are involved. The reduction of sample sizes could
solve this problem.
3. Improper choice of chromatographic systems such as the layer as well as
solvent system (stationary and mobile phases).
Tailed spots Ideal round spots spots
Fig. 10: Tailing in TLC
Applications of thin layer chromatography
Qualitative applications of TLC .1
The main use of TLC is qualitative analysis of substances whether in pure form or
in mixtures. TLC is a valuable tool to the extent that it is almost indispensable in all
chemistry laboratories (organic, biochemistry, analytical and natural products
chemistry). These applications can be illustrated in the following areas:
(a) Identification of substances: This can be accomplished either by
cochromatographing the test material alongside reference materials. Then by
comparing Rf values as well as color of spots of test and reference materials using
at least two different solvent systems. Alternatively, Rf values are rarely compared
to those in tables.
126