Page 23 - Pharmaceutical analytical chemistry |
P. 23
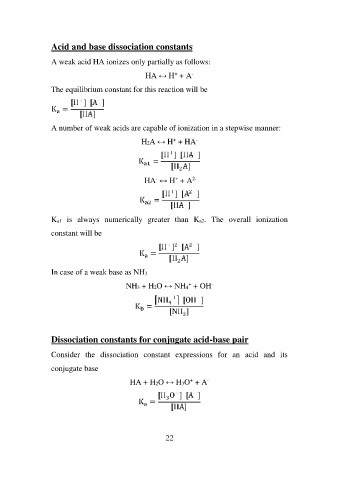
Acid and base dissociation constants
A weak acid HA ionizes only partially as follows:
HA ↔ H+ + A-
The equilibrium constant for this reaction will be
A number of weak acids are capable of ionization in a stepwise manner:
H2A ↔ H+ + HA-
HA- ↔ H+ + A2-
Ka1 is always numerically greater than Ka2. The overall ionization
constant will be
In case of a weak base as NH3
NH3 + H2O ↔ NH4+ + OH-
Dissociation constants for conjugate acid-base pair
Consider the dissociation constant expressions for an acid and its
conjugate base
HA + H2O ↔ H3O+ + A-
22