Page 25 - Pharmaceutical analytical chemistry |
P. 25
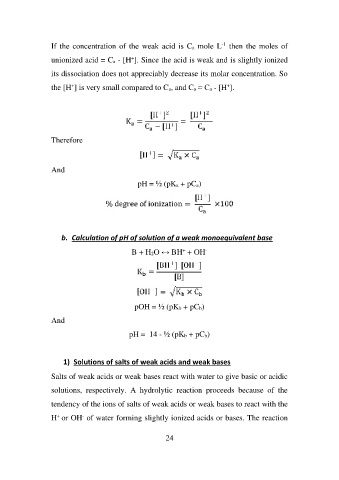
If the concentration of the weak acid is Ca mole L-1 then the moles of
unionized acid = Ca - [H+]. Since the acid is weak and is slightly ionized
its dissociation does not appreciably decrease its molar concentration. So
the [H+] is very small compared to Ca, and Ca ≈ Ca - [H+].
Therefore
And
pH = ½ (pKa + pCa)
b. Calculation of pH of solution of a weak monoequivalent base
B + H2O ↔ BH+ + OH-
pOH = ½ (pKb + pCb)
And
pH = 14 - ½ (pKb + pCb)
1) Solutions of salts of weak acids and weak bases
Salts of weak acids or weak bases react with water to give basic or acidic
solutions, respectively. A hydrolytic reaction proceeds because of the
tendency of the ions of salts of weak acids or weak bases to react with the
H+ or OH- of water forming slightly ionized acids or bases. The reaction
24