Page 40 - Biochemistry2 (08PB403)
P. 40
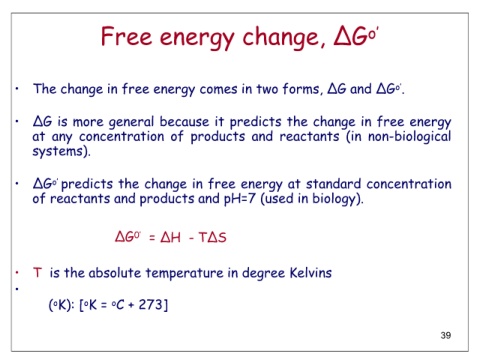
Free energy change, ΔGo’
• The change in free energy comes in two forms, ΔG and ΔGo’.
• ΔG is more general because it predicts the change in free energy
at any concentration of products and reactants (in non-biological
systems).
• ΔGo’ predicts the change in free energy at standard concentration
of reactants and products and pH=7 (used in biology).
ΔG0’ = ΔH - TΔS
• T is the absolute temperature in degree Kelvins
•
(oK): [oK = oC + 273]
39