Page 81 - phytochemistry II -pharmD general
P. 81
2- Mandalin’s reagent ⎯→ violet –blue color.
3-Pure strychnine dissolves in conc. HNO3 with a yellow color.
Brucine
1- Conc. HNO3 ⎯→ red color turning violet when adding drops of freshly prepared
solution of stannous chloride.
Estimation of Nux-vomica alkaloids:
Volumetric estimation
The total alkaloids are estimated by titrating the total alkaloidal extract
against acid. For the estimation of strychnine only, the titration product is oxidized
with nitric acid (under controlled condition) so as to oxidize only the alkaloid
brucine. The unoxidised strychnine is then extracted, and titrated against acid.
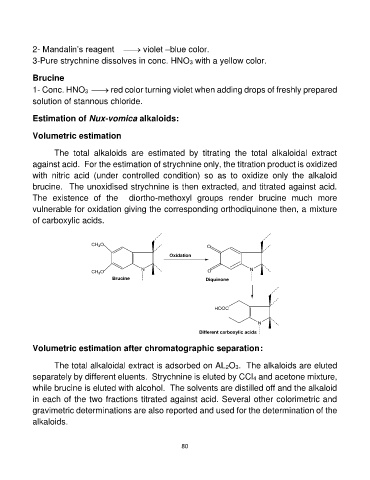
The existence of the diortho-methoxyl groups render brucine much more
vulnerable for oxidation giving the corresponding orthodiquinone then, a mixture
of carboxylic acids.
CH3O O
Oxidation O
Diquinone
CH3O N N
Brucine
HOOC
N
Different carboxylic acids
Volumetric estimation after chromatographic separation:
The total alkaloidal extract is adsorbed on AL2O3. The alkaloids are eluted
separately by different eluents. Strychnine is eluted by CCl4 and acetone mixture,
while brucine is eluted with alcohol. The solvents are distilled off and the alkaloid
in each of the two fractions titrated against acid. Several other colorimetric and
gravimetric determinations are also reported and used for the determination of the
alkaloids.
80