Page 77 - phytochemistry II -pharmD general
P. 77
OH 6 OH
7 NMe O 8 NMe
H H
O8
54
3
2 N
H
N1 (+)-Isolysergic acid
H
(+)-Lysergic acid
NH3 NH3
NH2
NH2 6
7
6 7 NMe
H
O 8 NMe O8
H
54
54
3
3
2 2
N1 N1
H H
Ergine Isoergine
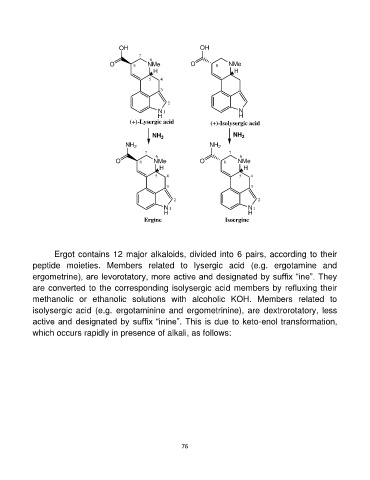
Ergot contains 12 major alkaloids, divided into 6 pairs, according to their
peptide moieties. Members related to lysergic acid (e.g. ergotamine and
ergometrine), are levorotatory, more active and designated by suffix “ine”. They
are converted to the corresponding isolysergic acid members by refluxing their
methanolic or ethanolic solutions with alcoholic KOH. Members related to
isolysergic acid (e.g. ergotaminine and ergometrinine), are dextrorotatory, less
active and designated by suffix “inine”. This is due to keto-enol transformation,
which occurs rapidly in presence of alkali, as follows:
76