Page 105 - Pharm.Org.Chem-I 02-06-05-101
P. 105
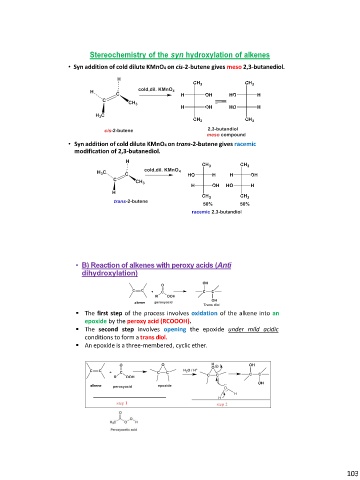
Stereochemistry of the syn hydroxylation of alkenes
• Syn addition of cold dilute KMnO4 on cis-2-butene gives meso 2,3-butanediol.
• Syn addition of cold dilute KMnO4 on trans-2-butene gives racemic
modification of 2,3-butanediol.
• B) Reaction of alkenes with peroxy acids (Anti
dihydroxylation)
▪ The first step of the process involves oxidation of the alkene into an
epoxide by the peroxy acid (RCOOOH).
▪ The second step involves opening the epoxide under mild acidic
conditions to form a trans diol.
▪ An epoxide is a three-membered, cyclic ether.
103