Page 7 - Pharm.Org.Chem-I 02-06-05-101
P. 7
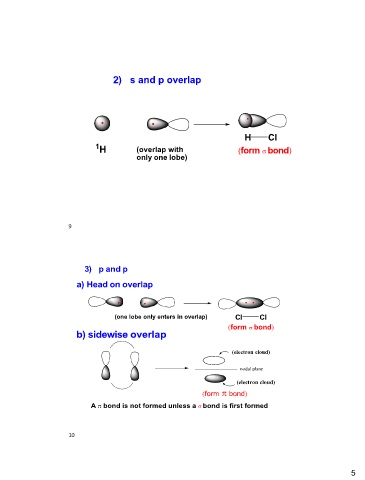
2) s and p overlap H Cl
(form bond)
..
1H (overlap with
only one lobe)
9
3) p and p ..
a) Head on overlap
Cl Cl
.. (form bond)
(one lobe only enters in overlap)
b) sidewise overlap
(electron cloud)
nodal plane
(electron cloud)
(form bond)
A bond is not formed unless a bond is first formed
10
5