Page 93 - Pharm.Org.Chem-I 02-06-05-101
P. 93
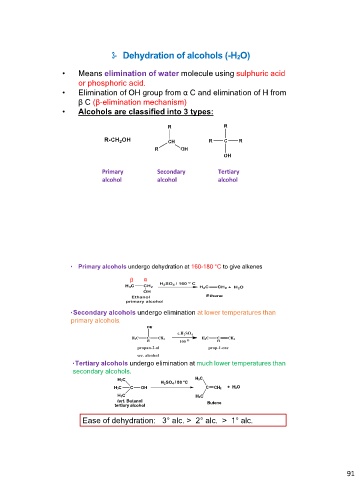
3- Dehydration of alcohols (-H2O)
• Means elimination of water molecule using sulphuric acid
or phosphoric acid.
• Elimination of OH group from α C and elimination of H from
β C (β-elimination mechanism)
• Alcohols are classified into 3 types:
Primary Secondary Tertiary
alcohol alcohol alcohol
• Primary alcohols undergo dehydration at 160-180 °C to give alkenes
βα
• Secondary alcohols undergo elimination at lower temperatures than
primary alcohols,
• Tertiary alcohols undergo elimination at much lower temperatures than
secondary alcohols.
H3C H2SO4 / 80 oC H3C
H3C C C CH2 + H2O
OH
H3C H3C
tert. Butanol Butene
tertiary alcohol
Ease of dehydration: 3° alc. > 2° alc. > 1° alc.
91