Page 57 - UKBM KIMIA XI Genap 2021
P. 57
Kim/3.12/4.12/4/1.2 17
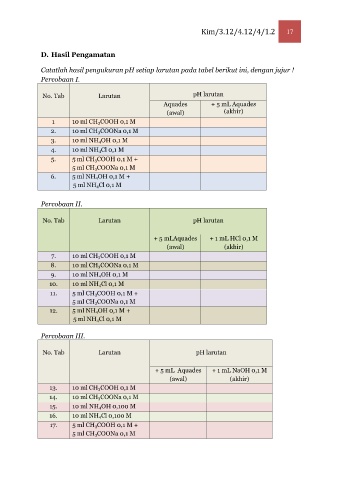
D. Hasil Pengamatan
Catatlah hasil pengukuran pH setiap larutan pada tabel berikut ini, dengan jujur !
Percobaan I.
No. Tab Larutan pH larutan
Aquades + 5 mL Aquades
(awal) (akhir)
1 10 ml CH3COOH 0,1 M
2. 10 ml CH3COONa 0,1 M
3. 10 ml NH4OH 0,1 M
4. 10 ml NH4Cl 0,1 M
5. 5 ml CH3COOH 0,1 M +
5 ml CH3COONa 0,1 M
6. 5 ml NH4OH 0,1 M +
5 ml NH4Cl 0,1 M
Percobaan II.
No. Tab Larutan pH larutan
+ 5 mLAquades + 1 mL HCl 0,1 M
(awal) (akhir)
7. 10 ml CH3COOH 0,1 M
8. 10 ml CH3COONa 0,1 M
9. 10 ml NH4OH 0,1 M
10. 10 ml NH4Cl 0,1 M
11. 5 ml CH3COOH 0,1 M +
5 ml CH3COONa 0,1 M
12. 5 ml NH4OH 0,1 M +
5 ml NH4Cl 0,1 M
Percobaan III.
No. Tab Larutan pH larutan
+ 5 mL Aquades + 1 mL NaOH 0,1 M
(awal) (akhir)
13. 10 ml CH3COOH 0,1 M
14. 10 ml CH3COONa 0,1 M
15. 10 ml NH4OH 0,100 M
16. 10 ml NH4Cl 0,100 M
17. 5 ml CH3COOH 0,1 M +
5 ml CH3COONa 0,1 M