Page 56 - PC 101 Interactive practical book
P. 56
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
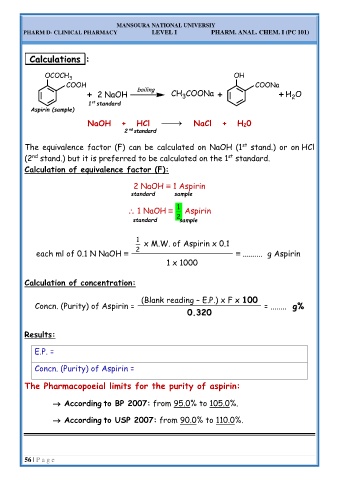
C Ca al lc cu ul la at ti io on ns s : :
1 st standard
Aspirin (sample)
NaOH + HCl NaCl + H 20
nd standard
2
The equivalence factor (F) can be calculated on NaOH (1 stand.) or on HCl
st
nd
(2 stand.) but it is preferred to be calculated on the 1 standard.
st
Calculation of equivalence factor (F):
2 NaOH ≡ 1 Aspirin
standard sample
1
1 NaOH ≡ Aspirin
standard 2 sample
1 x M.W. of Aspirin x 0.1
each ml of 0.1 N NaOH ≡ 2 ≡ .......... g Aspirin
1 x 1000
Calculation of concentration:
(Blank reading – E.P.) x F x 1 10 00 0
Concn. (Purity) of Aspirin = = ........ g g% %
0 0. .3 32 20 0
Results:
E.P. =
Concn. (Purity) of Aspirin =
The Pharmacopoeial limits for the purity of aspirin:
According to BP 2007: from 95.0% to 105.0%.
According to USP 2007: from 90.0% to 110.0%.
56 | P a g e