Page 61 - PC 101 Interactive practical book
P. 61
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
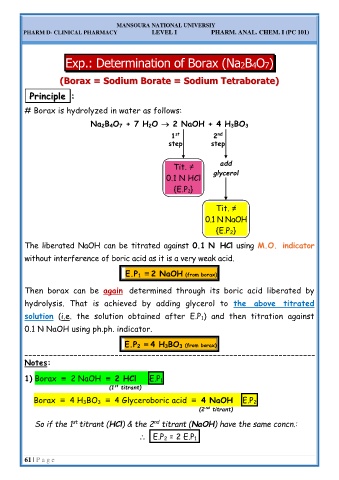
Exp.: Determination of Borax (Na2B4O7)
m Borate = Sodium Tetraborate)
( (B Bo or ra ax x = = S So od di iu um Borate = Sodium Tetraborate)
P Pr ri in nc ci ip pl le e : :
# Borax is hydrolyzed in water as follows:
Na 2B 4O 7 + 7 H 2O 2 NaOH + 4 H 3BO 3
nd
1
st
2
step step
Tit. ≠ add
0.1 N HCl glycerol
{E.P 1}
Tit. ≠
0.1 N NaOH
{E.P 2}
The liberated NaOH can be titrated against 0.1 N HCl using M.O. indicator
without interference of boric acid as it is a very weak acid.
E.P 1 ≡ 2 NaOH (from borax)
Then borax can be again determined through its boric acid liberated by
hydrolysis. That is achieved by adding glycerol to the above titrated
solution (i.e. the solution obtained after E.P 1) and then titration against
0.1 N NaOH using ph.ph. indicator.
E.P 2 ≡ 4 H 3BO 3 (from borax)
Notes:
1) Borax ≡ 2 NaOH ≡ 2 HCl E.P 1
(1 titrant)
st
Borax ≡ 4 H 3BO 3 ≡ 4 Glyceroboric acid ≡ 4 NaOH E.P 2
nd
(2 titrant)
st
nd
So if the 1 titrant (HCl) & the 2 titrant (NaOH) have the same concn.:
E.P 2 = 2 E.P 1.
61 | P a g e