Page 64 - Lab Manual & Project class 12
P. 64
LABORATORY MANUAL CHEMISTRY
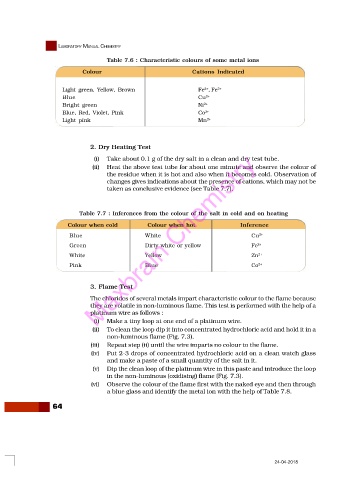
Table 7.6 : Characteristic colours of some metal ions
Colour Cations Indicated
2+
Light green, Yellow, Brown Fe , Fe 3+
Blue Cu 2+
Bright green Ni 2+
Blue, Red, Violet, Pink Co 2+
Light pink Mn 2+
2. Dry Heating Test
(i) Take about 0.1 g of the dry salt in a clean and dry test tube.
(ii) Heat the above test tube for about one minute and observe the colour of
the residue when it is hot and also when it becomes cold. Observation of
changes gives indications about the presence of cations, which may not be
taken as conclusive evidence (see Table 7.7).
Table 7.7 : Inferences from the colour of the salt in cold and on heating
Colour when cold Colour when hot Inference
Blue White Cu 2+
Green Maxbrain Chemistry Fe 2+
Dirty white or yellow
White Yellow Zn 2+
Pink Blue Co 2+
3. Flame Test
The chlorides of several metals impart characteristic colour to the flame because
they are volatile in non-luminous flame. This test is performed with the help of a
platinum wire as follows :
(i) Make a tiny loop at one end of a platinum wire.
(ii) To clean the loop dip it into concentrated hydrochloric acid and hold it in a
non-luminous flame (Fig. 7.3).
(iii) Repeat step (ii) until the wire imparts no colour to the flame.
(iv) Put 2-3 drops of concentrated hydrochloric acid on a clean watch glass
and make a paste of a small quantity of the salt in it.
(v) Dip the clean loop of the platinum wire in this paste and introduce the loop
in the non-luminous (oxidising) flame (Fig. 7.3).
(vi) Observe the colour of the flame first with the naked eye and then through
a blue glass and identify the metal ion with the help of Table 7.8.
64
24-04-2018