Page 66 - Lab Manual & Project class 12
P. 66
LABORATORY MANUAL CHEMISTRY
On heating, borax loses its water of crystallisation and decomposes to give
sodium metaborate and boric anhydride.
Na B O .10H O Na B O + 10H O
2 4 7 2 2 4 7 2
Borax
Na B O 7 2NaBO 2 + B O 3
2
2
4
Sodium metaborate Boric anhydride
On treatment with metal salt, boric anhydride forms metaborate of the metal
which gives different colours in oxidising and reducing flame. For example, in
the case of copper sulphate, following reactions occur.
CuSO + B O Non-luminous flame Cu(BO ) + SO
4 2 3 2 2 3
Cupric metaborate
Blue-green
Maxbrain Chemistry
Two reactions may take place in the reducing flame:
(i) The blue Cu (BO ) is reduced to colourless cuprous metaborate as follows:
2 2
2Cu(BO ) + 2NaBO + C Luminous flame 2CuBO + Na B O + CO
7
4
2
2
2
2 2
or (ii) Cupric metaborate may be reduced to metallic copper and the bead appears
red and opaque.
2Cu(BO ) + 4NaBO + 2C Luminous flame 2Cu + 2Na B O + 2CO
2
4
7
2 2
2
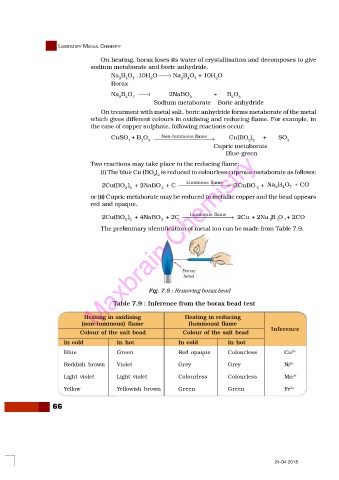
The preliminary identification of metal ion can be made from Table 7.9.
Fig. 7.5 : Removing borax bead
Table 7.9 : Inference from the borax bead test
Heating in oxidising Heating in reducing
(non-luminous) flame (luminous) flame
Inference
Colour of the salt bead Colour of the salt bead
In cold In hot In cold In hot
Blue Green Red opaque Colourless Cu 2+
Reddish brown Violet Grey Grey Ni 2+
Light violet Light violet Colourless Colourless Mn 2+
Yellow Yellowish brown Green Green Fe 3+
66
24-04-2018