Page 22 - P4304.1-V93_PS-Magazine-January 2024 -
P. 22
®
Cipla Ryaltris is indicated in adults and adolescents
12 years of age and older for the treatment of
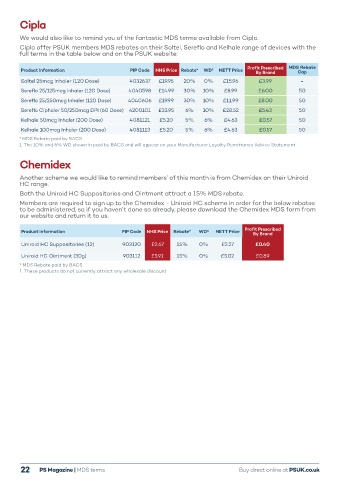
We would also like to remind you of the fantastic MDS terms available from Cipla. moderate to severe nasal symptoms associated
1
Cipla offer PSUK members MDS rebates on their Soltel, Sereflo and Kelhale range of devices with the with allergic rhinitis (AR).
full terms in the table below and on the PSUK website: Live like you
Profit Prescribed MDS Rebate
Product Information PIP Code NHS Price Rebate* WD¹ NETT Price By Brand Cap
Soltel 25mcg Inhaler (120 Dose) 4032637 £19.95 20% 0% £15.96 £3.99 - Ensure your practice takes full advantage of
Sereflo 25/125mcg Inhaler (120 Dose) 4040598 £14.99 30% 10% £8.99 £6.00 50 the Glenmark Dispensing Doctor Scheme
Sereflo 25/250mcg Inhaler (120 Dose) 4040606 £19.99 30% 10% £11.99 £8.00 50
Sereflo Ciphaler 50/250mcg DPI (60 Dose) 4200101 £33.95 6% 10% £28.52 £5.43 50
For Ryaltris, this means a 25% discount vs. NHS Drug Tariff
Kelhale 50mcg Inhaler (200 Dose) 4081121 £5.20 5% 6% £4.63 £0.57 50
Kelhale 100mcg Inhaler (200 Dose) 4081113 £5.20 5% 6% £4.63 £0.57 50 to boost your practice’s overall profit
* MDS Rebate paid by BACS
1. The 10% and 6% WD shown in paid by BACS and will appear on your Manufacturer Loyalty Remittance Advice Statement
To maximise your practice’s profit from this scheme, prescribe
Chemidex Ryaltris generically and dispense by brand:
Another scheme we would like to remind members’ of this month is from Chemidex on their Uniroid Ryaltris (olopatadine/mometasone furoate 600mcg/25mcg) nasal spray
HC range.
Both the Uniroid HC Suppositories and Ointment attract a 15% MDS rebate. PIP NHS Drug Manufacturer Net NHS Clawback Clawback Profit Profit
Members are required to sign up to the Chemidex - Uniroid HC scheme in order for the below rebates code indicative Tariff discount indicative vs. NHS vs. Drug vs. NHS vs. Drug
to be administered, so if you haven’t done so already, please download the Chemidex MDS form from price price price price Tariff price Tariff
our website and return it to us. 4190278 £13.32 £13.32 25% £9.99 £1.49 £1.49 £1.84 £1.84
Product Information PIP Code NHS Price Rebate* WD¹ NETT Price Profit Prescribed Wholesaler Discount not included and should be added to determine total profit.
By Brand
Prices taken from BNF, November 2023 2
Uniroid HC Suppositories (12) 903120 £2.67 15% 0% £2.27 £0.40
Uniroid HC Ointment (30g) 903112 £5.91 15% 0% £5.02 £0.89 Find out more at www.ryaltris.co.uk Scan to visit
* MDS Rebate paid by BACS www.ryaltris.co.uk
1. These products do not currently attract any wholesale discount
Ryaltris Prescribing Information during periods of stress or elective surgery. The replacement of a systemic corticosteroid
Ryaltris (mometasone furoate and olopatadine hydrochloride) Please refer to the with a topical corticosteroid can be accompanied by signs of adrenal insufficiency, and
Summary of Product Characteristics (SmPC) before prescribing. One delivered dose some patients may experience symptoms of withdrawal. Patients previously treated for
contains mometasone furoate monohydrate equivalent to 25 microgram mometasone prolonged periods with systemic corticosteroids and transferred to topical corticosteroids
furoate and olopatadine hydrochloride equivalent to 600 micrograms olopatadine. should be carefully monitored for acute adrenal insufficiency in response to stress.
Indication: treatment of moderate to severe nasal symptoms associated with allergic In those patients who have asthma or other clinical conditions requiring long term
rhinitis in adults and adolescents 12 years of age and older Posology and method of systemic corticosteroid treatment, too rapid a decrease in systemic corticosteroids may
administration: The usual recommended dose is two actuations in each nostril twice cause a severe exacerbation of their symptoms. Somnolence: in isolated cases dizziness,
daily (morning and evening). Children below 12 years: Ryaltris is not recommended. lethargy, fatigue and somnolence may occur when using Ryaltris. In these cases, the
Elderly: No dose adjustment required. Renal and hepatic impairment: there are no data ability to drive and use machines may be impaired. Alcohol and other CNS depressants
in patients with renal and hepatic impairment, however no dose adjustment is expected may enhance this effect. Antihistamine effects: concomitant use of other antihistaminic
to be required. Contraindications: Hypersensitivity to the active substances or to any of drugs administered may increase the risk of antihistamine adverse effects. Paediatric
the excipients. Ryaltris should not be used in the presence of untreated localised infection population: It is recommended that the height of children receiving prolonged treatment
involving the nasal mucosa, such as herpes simplex. Patients who have experienced recent with nasal corticosteroids is regularly monitored. If growth is slowed, therapy should be
nasal surgery or trauma should not use a nasal corticosteroid until healing has occurred. reviewed with the aim of reducing the dose of nasal corticosteroid if possible, to the lowest
Precautions: Local nasal effects: nasal ulceration and nasal septal perforation have been dose at which effective control of symptoms is maintained. Excipients: Ryaltris contains
reported in patients following intranasal antihistamines. Nasal septal perforation has been 0.02 mg benzalkonium chloride in each actuation. Benzalkonium chloride may cause
reported following intranasal corticosteroids. Patients using Ryaltris over several months irritation or swelling inside the nose, especially if used for a long time. Adverse reactions:
or longer should be examined periodically for possible changes in the nasal mucosa. Common (≥1/100 to <1/10): dysgeusia (unpleasant taste), epistaxis, nasal discomfort.
Ryaltris is not recommended in cases of nasal septum perforation. In clinical studies with Uncommon (≥1/1,000 to <1/100): dizziness, headaches, somnolence, nasal dryness, dry
mometasone furoate administered intranasally, the development of localised infections mouth, abdominal pain, nausea, fatigue. Rare (≥1/10,000 to <1/1000) bacterial vaginosis,
of the nose and pharynx with Candida albicans has occurred; may require treatment and anxiety, depression, insomnia, lethargy, migraine, blurred vision, dry eye, eye discomfort,
discontinuation of Ryaltris. Patients using Ryaltris over several months or longer should be ear pain, nasal inflammation, nasal mucosal disorder, oropharyngeal pain, sneezing,
examined periodically for evidence of Candida infection or other signs of adverse effects throat irritation, constipation, sore tongue, laceration. Incidence not known (reported
on the nasal mucosa. Visual disturbances may be reported with systemic and topical from use of corticosteroids): pharyngitis, upper respiratory tract infection, hypersensitivity
corticosteroid use. Symptoms such as blurred vision or other visual disturbances should including anaphylactic reactions, angioedema, bronchospasm, and dyspnoea, cataracts,
be considered for ophthalmologist referral for evaluation of possible causes including glaucoma, increased intraocular pressure, nasal septum perforation. Marketing
cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR). authorisation number: PL 25258/0331 Marketing Authorisation Holder and distributer:
Hypersensitivity Reactions including instances of wheezing, may occur. Discontinue Glenmark Pharmaceuticals Europe Limited, Laxmi House, 2B Draycott Avenue
Ryaltris if such reactions occur. Immunosuppression: Chickenpox and measles, for Kenton, Middlesex, HA3 0BU. United Kingdom Legal classification: POM Cost: £13.32.
example, can have a more serious or even fatal course in susceptible patients and those 1 bottle with 29 g suspension (240 actuations) Date of preparation: June 2021.
using corticosteroids. In children or adults who have not had these diseases or been Job number: PP-UK-RYAL-0001
properly immunized, particular care should be taken to avoid exposure. Corticosteroids
should be used with caution, if at all, in patients with active or quiescent tuberculous
infections of the respiratory tract, untreated local or systemic fungal or bacterial infections, Adverse events should be reported. Reporting forms and information
systemic viral or parasitic infections, or ocular herpes simplex because of the potential can be found at https://yellowcard.mhra.gov.uk. Adverse events should
for worsening of these infections. Systemic Effects of Corticosteroids: potential systemic also be reported to Glenmark Pharmaceuticals Europe Ltd
effects may include Cushing’s syndrome, Cushingoid features, adrenal suppression, medical_information@glenmarkpharma.com or call 0800 458 0383
growth retardation in children and adolescents, cataract, glaucoma and more rarely, a
range of psychological or behavioural effects including psychomotor hyperactivity, sleep
disorders, anxiety, depression or aggression (particularly in children). When intranasal References: 1. Ryaltris Summary of Product Characteristics. 2. British National Formulary.
steroids are used at higher than recommended dosages or in susceptible individuals Available at: https://bnf.nice.org.uk/drugs/mometasone-furoate-with-olopatadine/
at recommended dosages, systemic corticosteroid effects such as hypercorticism and medicinal-forms/#spray.
adrenal suppression may appear. If such changes occur, the dosage of Ryaltris should
be discontinued slowly, consistent with accepted procedures for discontinuing oral Ryaltris is a registered trademark of Glenmark Speciality S.A.
®
corticosteroid therapy. The concomitant use of intranasal corticosteroids with other
inhaled corticosteroids could increase the risk of signs or symptoms of hypercorticism © 2023 Glenmark Pharmaceuticals Europe Ltd.
and/or suppression of the HPA axis. If there is evidence for higher than recommended All rights reserved.
doses being used, then additional systemic corticosteroid cover should be considered Date of preparation: December 2023 PP-UK-RYAL-0338 V2
22 PS Magazine | MDS terms Buy direct online at PSUK.co.uk
P4304.1-V93_PS-Magazine-January 2024.indd 22 14/12/2023 10:11:25
P4304.1-V93_PS-Magazine-January 2024.indd 22
14/12/2023 10:11:25
04/12/2023 09:40
020040_AW1_Ryaltris_PSUK_Print_Ad_210x297mm.indd 1 04/12/2023 09:40
020040_AW1_Ryaltris_PSUK_Print_Ad_210x297mm.indd 1