Page 28 - Practical-organic-3
P. 28
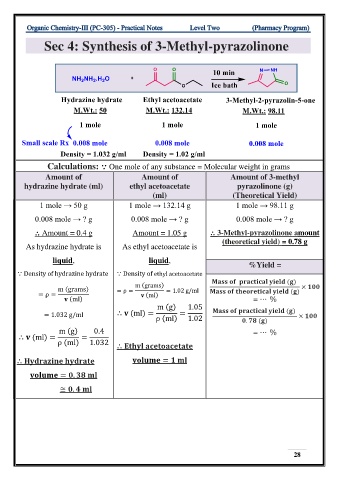
Sec 4: Synthesis of 3-Methyl-pyrazolinone
Calculations: ⸪ One mole of any substance = Molecular weight in grams
Amount of Amount of Amount of 3-methyl
hydrazine hydrate (ml) ethyl acetoacetate pyrazolinone (g)
(ml) (Theoretical Yield)
1 mole → 50 g 1 mole → 132.14 g 1 mole → 98.11 g
0.008 mole → ? g 0.008 mole → ? g 0.008 mole → ? g
⸫ Amount = 0.4 g Amount = 1.05 g ⸫ 3-Methyl-pyrazolinone amount
(theoretical yield) = 0.78 g
As hydrazine hydrate is As ethyl acetoacetate is
liquid, liquid,
%Yield =
⸪ Density of hydrazine hydrate ⸪ Density of ethyl acetoacetate
( )
m (grams) ×
m (grams) = ρ = = 1.02 g/ml ( )
= ρ = (ml)
(ml) = ⋯ %
m (g) 1.05 ( )
= 1.032 g/ml ⸫ (ml) = = ×
ρ (ml) 1.02 . ( )
m (g) 0.4 = ⋯ %
⸫ (ml) = =
ρ (ml) 1.032
⸫
⸫ =
= .
≅ .
28