Page 40 - Practical-organic-3
P. 40
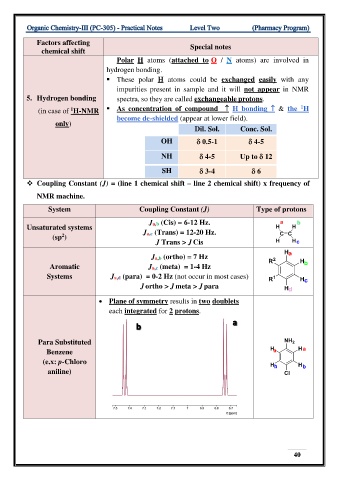
Factors affecting
chemical shift Special notes
Polar H atoms (attached to O / N atoms) are involved in
hydrogen bonding.
▪ These polar H atoms could be exchanged easily with any
impurities present in sample and it will not appear in NMR
5. Hydrogen bonding spectra, so they are called exchangeable protons.
1
1
(in case of H-NMR ▪ As concentration of compound H bonding & the H
become de-shielded (appear at lower field).
only)
Dil. Sol. Conc. Sol.
OH 0.5-1 4-5
NH 4-5 Up to 12
SH 3-4 6
❖ Coupling Constant (J) = (line 1 chemical shift – line 2 chemical shift) x frequency of
NMR machine.
System Coupling Constant (J) Type of protons
Ja,b (Cis) = 6-12 Hz.
Unsaturated systems Ja,c (Trans) = 12-20 Hz.
2
(sp )
J Trans > J Cis
Ja,b (ortho) = 7 Hz
Aromatic Ja,c (meta) = 1-4 Hz
Systems Ja,d (para) = 0-2 Hz (not occur in most cases)
J ortho > J meta > J para
• Plane of symmetry results in two doublets
each integrated for 2 protons.
a
b
Para Substituted
Benzene
(e.x: p-Chloro
aniline)
40