Page 45 - Practical-organic-3
P. 45
❖ Complete the following statements:
1. Rank the following molecules in the order of the one with most de-shielded proton(s)
to the one with least de-shielded proton(s) (CH3, CH2, CH4, CH)
………>………>………>………
2. Higher electronegativity of the atom that the proton is attached to, the proton
is…………..shielded; the proton has a………….chemical shift.
3. If a proton is more shielded, then it has …………..chemical shift.
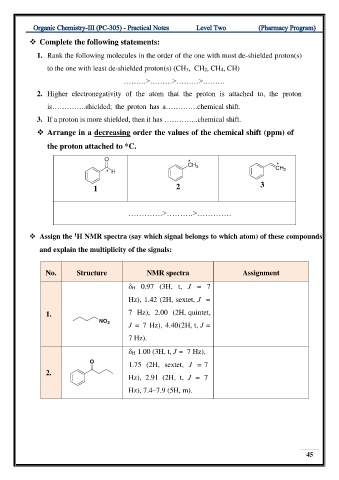
❖ Arrange in a decreasing order the values of the chemical shift (ppm) of
the proton attached to *C.
3
1 2
………….>……….>………….
1
❖ Assign the H NMR spectra (say which signal belongs to which atom) of these compounds
and explain the multiplicity of the signals:
No. Structure NMR spectra Assignment
H 0.97 (3H, t, J = 7
Hz), 1.42 (2H, sextet, J =
1. 7 Hz), 2.00 (2H, quintet,
J = 7 Hz), 4.40 (2H, t, J =
7 Hz).
H 1.00 (3H, t, J = 7 Hz),
1.75 (2H, sextet, J = 7
2.
Hz), 2.91 (2H, t, J = 7
Hz), 7.4–7.9 (5H, m).
45