Page 72 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 72
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
CH 3COOH CH 3COO + H
+
-
+
• If more H is added to this solution, it simply shifts the equilibrium to the
left, absorbing H , so the [H ] remains unchanged.
+
+
+
-
• If H is removed (e.g. by adding OH ) then the equilibrium shifts to the right,
+
releasing H to keep the pH constant.
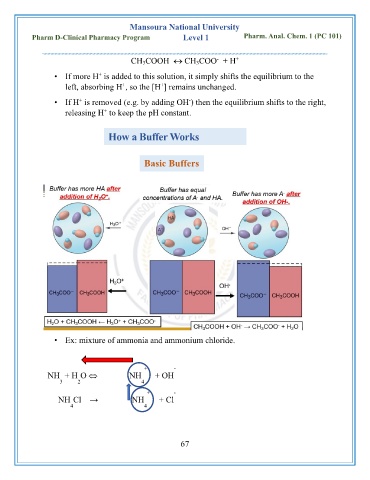
How a Buffer Works
Basic Buffers
• Ex: mixture of ammonia and ammonium chloride.
+ -
NH + H O NH + OH
3 2 4
+ -
NH Cl → NH + Cl
4 4
67