Page 507 - Physics Coursebook 2015 (A level)
P. 507
Chapter 31: Nuclear physics
16
20 Ne
10 35Cl
168O 17 1 62 C
4 147N 2He 115B
9Be
21H
63 Li
19 F
56Fe 26
75 As 33
100Mo 126 Te 42 52
89Y 110
39 48Cd
141Pr 59
160Dy 66
180Hf 72
197 Au 79
209Bi 83
238U 92
12
4
8
4
0
0 40 80 120 160 200 240
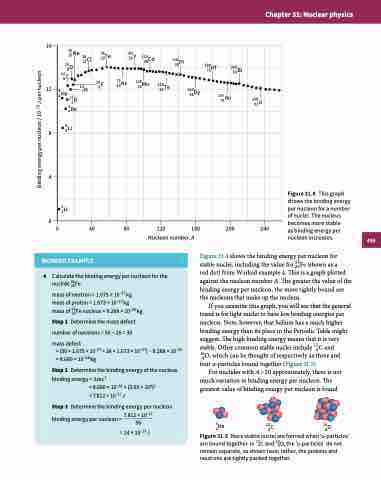
Figure 31.4 This graph shows the binding energy per nucleon for a number of nuclei. The nucleus becomes more stable
as binding energy per nucleon increases.
Nucleon number, A
WORKED EXAMPLE
Figure 31.4 shows the binding energy per nucleon for stable nuclei, including the value for 56Fe (shown as a
4 Calculate the binding energy per nucleon for the nuclide 56Fe.
26
red dot) from Worked example 4. This is a graph plotted
against the nucleon number A. The greater the value of the binding energy per nucleon, the more tightly bound are the nucleons that make up the nucleus.
If you examine this graph, you will see that the general trend is for light nuclei to have low binding energies per nucleon. Note, however, that helium has a much higher binding energy than its place in the Periodic Table might suggest. The high binding energy means that it is very stable. Other common stable nuclei include 126C and
186O, which can be thought of respectively as three and four α-particles bound together (Figure 31.5).
For nuclides with A > 20 approximately, there is not much variation in binding energy per nucleon. The greatest value of binding energy per nucleon is found
42He 126C 168O
Figure 31.5 More stable nuclei are formed when ‘α-particles’ are bound together. In 162C and 186O, the ‘α-particles’ do not remain separate, as shown here; rather, the protons and neutrons are tightly packed together.
26
mass of neutron = 1.675 × 10−27 kg
mass of proton = 1.673 × 10−27 kg
mass of 56Fe nucleus = 9.288 × 10−26 kg 26
Step1 Determinethemassdefect.
number of neutrons = 56 − 26 = 30
mass defect
=(30×1.675×10−27 +26×1.673×10−27)−9.288×10−26 = 8.680 × 10−28 kg
Step2 Determinethebindingenergyofthenucleus. binding energy = Δmc 2
= 8.680 × 10−28 × (3.00 × 108)2 =7.812×10−11J
Step3 Determinethebindingenergypernucleon. binding energy per nucleon = 7.812 × 10−11
56
≈ 14 × 10−13 J
495
Binding energy per nucleon / 10–13 J per nucleon