Page 47 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 47
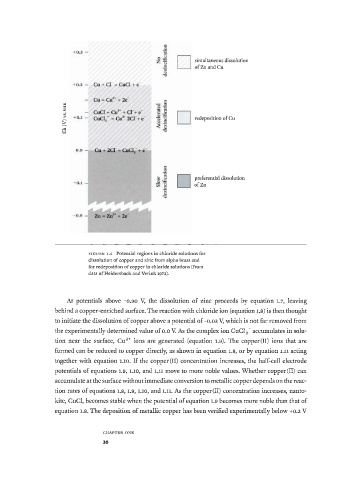
simultaneous dissolution
of Zn and Cu
tí
χ
> redeposition of Cu
>
tí
preferential dissolution
of Zn
F I G U R E 1.4 Potential regions in chloride solutions for
dissolution of copper and zinc from alpha brass and
for redeposition of copper in chloride solutions (from
data of Heidersbach and Verink 1972).
At potentials above -0.90 V, the dissolution of zinc proceeds by equation 1.7, leaving
behind a copper-enriched surface. The reaction with chloride ion (equation 1.8) is then thought
to initiate the dissolution of copper above a potential of-0.03 V, which is not far removed from
the experimentally determined value of 0.0 V. As the complex ion CuCl 2 ~ accumulates in solu
tion near the surface, C u 2 + ions are generated (equation 1.9). The copper(II) ions that are
formed can be reduced to copper directly, as shown in equation 1.8, or by equation 1.11 acting
I
together with equation 1.10. f the copper (II) concentration increases, the half-cell electrode
potentials of equations 1.9, 1.10, and 1.11 move to more noble values. Whether copper (II) can
accumulate at the surface without immediate conversion to metallic copper depends on the reac
tion rates of equations 1.8,1.9,1.10, and 1.11. As the copper (II) concentration increases, nanto-
kite, CuCl, becomes stable when the potential of equation 1.9 becomes more noble than that of
equation 1.8. The deposition of metallic copper has been verified experimentally below +0.2 V
C H A P T E R O N E
30