Page 51 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 51
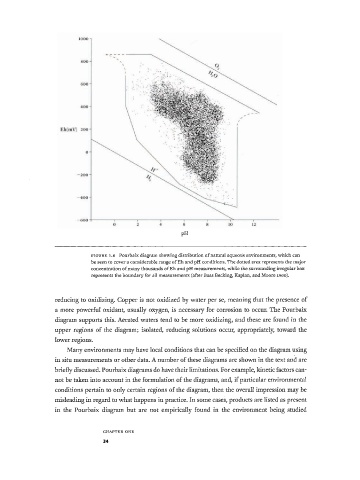
Pourbaix diagram showing distribution of natural aqueous environments, which can
F I G U R E 1.6
be seen to cover a considerable range of Eh and pH conditions. The dotted area represents the major
concentration of many thousands of Eh and pH measurements, while the surrounding irregular box
represents the boundary for all measurements (after Baas Becking, Kaplan, and Moore i960) .
reducing to oxidizing. Copper is not oxidized by water per se, meaning that the presence of
a more powerful oxidant, usually oxygen, is necessary for corrosion to occur. The Pourbaix
diagram supports this. Aerated waters tend to be more oxidizing, and these are found in the
upper regions of the diagram; isolated, reducing solutions occur, appropriately, toward the
lower regions.
Many environments may have local conditions that can be specified on the diagram using
in situ measurements or other data. A number of these diagrams are shown in the text and are
briefly discussed. Pourbaix diagrams do have their limitations. For example, kinetic factors can
not be taken into account in the formulation of the diagrams, and, f particular environmental
i
conditions pertain to only certain regions of the diagram, then the overall impression may be
misleading in regard to what happens in practice. In some cases, products are listed as present
in the Pourbaix diagram but are not empirically found in the environment being studied
C H A P T E R O N E
34