Page 67 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 67
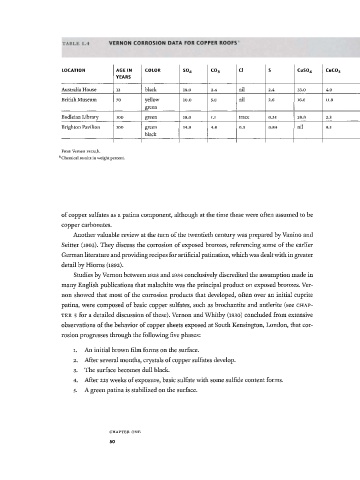
TABLE 1.4 VERNON CORROSION DATA FOR COPPER ROOFS
LOCATION AGE IN COLOR S 0 4 co 3 CI S CuS0 4 C11CO3
YEARS
Australia House 12 black 19.9 2.4 nil 2.4 33.0 4.9
British Museum 70 yellow 10.0 5.8 nil 2.6 16.6 11.9
green
Bodleian Library 100 green 18.0 1.1 trace 0.35 28.0 2.3
Brighton Pavilion 100 green 14.9 4.6 0.3 0.84 nil 9.5
black
From Vernon i932a,b.
Chemical results in weight percent.
of copper sulfates as a patina component, although at the time these were often assumed to be
copper carbonates.
Another valuable review at the turn of the twentieth century was prepared by Vanino and
Seitter (1903). They discuss the corrosion of exposed bronzes, referencing some of the earlier
German literature and providing recipes for artificial patination, which was dealt with in greater
detail by Hiorns (1892).
Studies by Vernon between 1923 and 1934 conclusively discredited the assumption made in
many English publications that malachite was the principal product on exposed bronzes. Ver
non showed that most of the corrosion products that developed, often over an initial cuprite
patina, were composed of basic copper sulfates, such as brochantite and anderite (see CHAP
TER 5 for a detailed discussion of these). Vernon and Whitby (1930) concluded from extensive
observations of the behavior of copper sheets exposed at South Kensington, London, that cor
rosion progresses through the following five phases:
1. An initial brown film forms on the surface.
2. After several months, crystals of copper sulfates develop.
3. The surface becomes dull black.
4. After 225 weeks of exposure, basic sulfate with some sulfide content forms.
5. A green patina is stabilized on the surface.
C H A P T E R O N E
50