Page 70 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 70
outlines their findings, which are presented here in the more helpful units of micrometers per
year of exposure:
0.5 μηι per year in rural atmospheres
l.o μπι per year in marine atmospheres
1-2 μπι per year in urban atmospheres
2.5 μπι per year in industrial atmospheres
In their own work, carried out over sixteen years, Holm and Mattsson (1982) exposed
thirty-six different copper alloys in sheet and rod form to atmospheric weathering at rural,
marine, and urban sites. Greenish patina formed on most of these alloys after six to seven years
at the urban and marine sites. At the rural site, however, no distinct green patina formed on any
alloy—only different shades of black or brown—after sixteen years of exposure. The amount
of patina retained on the copper alloy surfaces increased substantially during the exposure
period from seven to sixteen years. The patina that developed was more protective in the marine
and rural environments than in the urban one. The average penetration levels of the copper
alloys were .3-0.5 μπι per year for the rural site; .5-0.9 μπι per year for the marine site; and
0
0
0.9-1.3 μπι per year for the urban site. Rates such as these apply only to the first few years of
exposure when a patina is starting to develop; once a corrosion film has formed, the rate of cor
rosion drops substantially, usually after the first ten years.
The usual minerals that form on these exposed copper alloys are copper sulfates, but in a
number of cases, the alloying elements themselves contribute to the patina. Zinc sulfate or
basic zinc sulfate form on some brasses, and lead sulfate or basic lead sulfates form on free-
cutting phosphor bronze and on some leaded brasses. In Holm and Mattson's work (1982), silica
formed on silicon bronze in marine atmospheres; copper phosphate on some of the phosphor
bronzes n urban atmospheres; copper arsenates on arsenical copper and on some arsenical
i
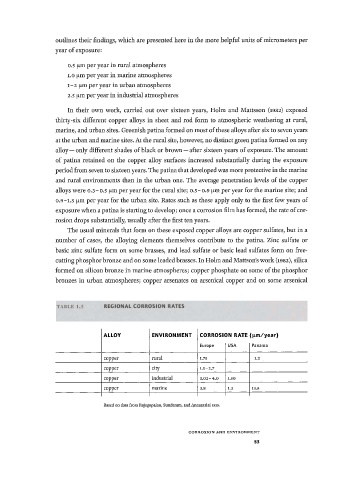
TABLE 1.5 REGIONAL CORROSION RATES
ALLOY ENVIRONMENT CORROSK DN RATE (μηι/year)
Europe USA Panama
copper rural 1.75 1.2
copper city 1.5-2.7
copper industrial 3 . 0 2 - 4 . 0 1.50
copper marine 3.8 1.3 13.8
Based on data from Rajagopalan, Sundaram, and Annamalai 1959.
C O R R O S I O N AND E N V I R O N M E N T
53