Page 5 - THEALOZ DUO LEAFLET CONGRES 2021_Slide
P. 5
100 p =0.044
76.9 11.5 FOR PATIENTS WITH DRY EYES 18.9 HA-trehalose
71.7
90 None: OSDI [0-13]
7.7 Mild: OSDI [13-23]
80 7.5 Moderate: OSDI [23-33]
19.2 MEDICAL DEVICE Severe: OSDI [33-100]
70
TREHALOSE 3% | HYALURONIC ACID 0.15%
34
% of patients 60 61.5 HA None: OSDI [0-13]
Mild: OSDI [13-23]
50
Better Patient Satisfaction 39.6 Moderate: OSDI [23-33]
40
Severe: OSDI [33-100]
30
22.6
20 19.2
10
0 3.8 5.7
D0 D84 D0 D84
GREATER IMPROVEMENT IN OSDI WITH THEALOZ DU VS HA 12 GREATER PATIENT SATISFACTION
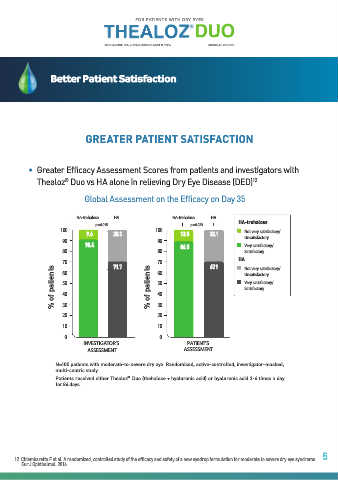
• Greater Efficacy Assessment Scores from patients and investigators with
Thealoz Duo vs HA alone in relieving Dry Eye Disease (DED) 12
®
Global Assessment on the Efficacy on Day 35
HA-trehalose HA HA-trehalose HA
p =0.015 p =0.023 HA-trehalose
100 100 Not very satisfactory/
9.6 28.3 13.5 32.1
90 90 Unsatisfactory
90.4 86.5 Very satisfactory/
80 80 Satisfactory
70 71.7 70 67.9 HA Not very satisfactory/
% of patients 50 % of patients 50 Very satisfactory/
60
60
Unsatisfactory
Satisfactory
40
40
30
20
20 30
10 10
0 0
INVESTIGATOR’S PATIENT’S
ASSESSMENT ASSESSMENT
N=105 patients with moderate-to-severe dry eye. Randomised, active-controlled, investigator-masked,
multi-centric study. HA-trehalose
HA
Patients received either Thealoz ® Duo (thehalose + hyaluronic acid) or hyaluronic acid 3-6 times a day
for 84 days.
*p =0.05
5
12. Chiambaretta F et al. A randomized, controlled study of the efficacy and safety of a new eyedrop formulation for moderate to severe dry eye syndrome.
Eur J Ophthalmol. 2016.