Page 96 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 96
Intravascular Mesenchymal Stem Cells to Treat Organ Failure and Possible Application in COVID-19
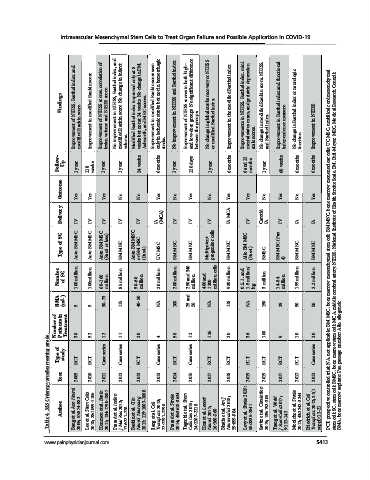
Improvement of NIHSS, Barthel index, and modified Rankin score. Improvement in modified Rankin score Improvement of NIHSS scores, correlation of lesion volume and NIHSS score. modified Rankin score. No change in infarct Modified Barthel index improved only at 8 weeks but not at 24 weeks. No change in FM, Ashworth, and MRC scores. Improvement in modified Rankin score seen Improvement of NIHSS scores in both high- between the groups. or modified
Findings No improvement in NIHSS, Barthel index, and only in ischemic stroke but not in hemorrhagic No improvement in NIHSS and Barthel index. and low-dose groups. No significant difference No change in global stroke recovery or NIHSS
Follow- Up 1 year 118 weeks 1 year 1 year volume. 24 weeks 6 months stroke. 1 year 120 days 1 year 6 months 6 and 12 months scale scores. 1 year 60 weeks 6 months function. 6 months
Outcome Yes Yes Yes No No Yes No Yes No Yes Yes No Yes No Yes
Delivery IV IV IV IV IV IA (MCA) IV IV IV IA MCA IV Carotid IA IV IA IA
Type of SC Auto BM MSC Auto BM MSC Auto BM MSC (Pas 3 or less) BM MNC Auto BM MNC or BM MSC (Pas 4) UC MSC BM MNC BM MNC Multipotent progenitor cells BM MNC Allo BM MSC (Pas 4) BMSC BM MSC (Pas 4) BM MNC BM MNC PCT, prospective controlled trial; NA, not applicable; BM MSC, bone marrow mesenchymal stem cell; BM MNC, bone marrow mononuclear cells; UC MSC, umbilical cord mesenchymal stem cell; SC, stem cell; BMSC, bone marrow stem cell; M
Number of SC 100 million 100million 60–160 million 85 million 50–60 million 20 million 280 million 250 and 340 million 400 and 1200 million cells 930 million 0.5, 1, and 1.5 million/ kg 3 million 14–84 million 159 million 2.2 million
BMA (mL) 5 5 30–73 115 40–50 NA 108 25 and 50 NA 118 NA 150 29 50 50
Number of Patients in Treatment 30 52 12 11 20 4 58 12 126 20 36 100 9 10 20
Table 4. MSC therapy studies treating stroke. Type of Year Author study Bang et al, Ann Neurol RCT 2005 Lee et al, Stem Cells RCT 2010 Honmou et al, Brain Case series 2011 2011; 134:1790-1807 Case series 2012 PCT 2013 2013; 115:1003-1008 Case series 2013 RCT 2014 Case series 2015 RCT 2017 RCT 2018 Levy et al, Stroke 2019; PCT 2019 Savitz et al, Circulation RCT 2019 RCT 2017 Moniche et al, Stroke PCT 2012 Case series 20
2010; 28:1099-1106
2005; 57:874-882
suppl):13-21
www.painphysicianjournal.com Prasad et al, Indian J Med Res 2012; 136:221-228 Bashin et al, Clin Neurol Neurosurg Jiang et al, Cell Transplant 2013; 22:2291-2298 Prasad et al, Stroke 2014; 45:3618-3624 Taguchi et al, Stem Cells Dev 2015; 24:2207-2218 Hess et al, Lancet Neurol 2017; 16:360-368 Bhatia et al, Am J Neuroradiol 2018; 39:899-904 50:2835-2841 2019; 139:192-205 Tsang et al, World J Stem Cells 2017; 9:133-143 2012; 43:22