Page 97 - Mesenchymal Stem cells, Exosomes and vitamins in the fight aginst COVID
P. 97
Pain Physician: August 2020 COVID-19 Special Issue 23:S391-S420
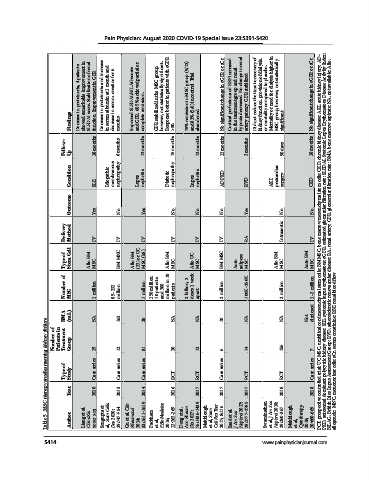
Decrease in proteinuria, 4 patients showed remarkable improvement in SLEDAI score. Stabilization of renal function. Improvement in GFR. Decrease in proteinuria and increase in serum albumin at 1 month and decrease in serum creatine for 6 Improved SLEDAI/BILAG scores and GFR, 60.5% achieved partial or GFR stabilized in the MSC group, however, not statistically significant. Response better in patients with eGFR 75% remission in MSC group (9/12)
Findings months. complete remission. >30. abandoned. significant.
Follow- Up 18 months 6 months 12 months 15 months 12 months 12 months 3 months 90 days 18 months
Condition SLE Idiopathic membranous nephropathy Lupus nephritis Diabetic nephropathy Lupus nephritis ADPKD RVD AKI postcardiac surgery CKD
Outcome Yes No Yes No No No Yes No No
Delivery Method IV IV IV IV IV IV RA Intraaortic IV PCT, prospective controlled trial; UC MSC, umbilical cord mesenchymal stem cells; BM MSC, bone marrow mesenchymal stem cells; CKD, chronic kidney disease; AKI, acute kidney injury; AD- PKD, autosomal dominant polycystic kidney disease; SLE, systemic lupus erythematous; eGFR, estimated glomerular filtration rate; SLEDAI, Systemic Lupus Erythematous Disease Activity Index; BIL
Type of Stem Cell Allo BM MSC BM MNC Allo BM (23) or UC MSC (58) Allo BM MSC Allo UC MSC BM MSC Auto adipose MSC Allo BM MSC Auto BM MSC
Number of MSC 1 million 85–232 million 1 million 150 million 10 patients and 300 million in 10 patients 2 billion, 2 doses 1 week apart 2 million 100K–250K 2 million 1–2 million
BMA (mL) NA 162 20 NA NA 10 NA NA Not disclosed
Table 5. MSC therapy studies treating kidney failure.
Number of Patients in Treatment Group 15 12 81 20 12 6 14 156 7 allogeneic; MNC, mononuclear cells; sCr, serum creatinine; RBF, renal blood flow.
Type of Study Case series Case series Case series RCT RCT Case series PCT RCT Case series
Year 2010 2013 2014 2016 2017 2017 2017 2018 2018
Author Liang et al, ChinaXiv 2020; 1-13 Sengupta et al, Stem Cells Dev 2020; 29:747-754 Gu et al, Clin Rheumatol 2014; 33:1611-1619 Packham et al, EBioMedicine 2016; 12:263-269 Deng et al, Ann Rheum Dis 2017; 76:1436-1439 Makhlough et al, Stem Cell Res Ther 2017; 8:116 Saad et al, J Am Soc Nephrol 2017; 28:2777-2785 Swaminathan et al, J Am Soc Nephrol 2018; 29:260-267 Makhlough et al, Cytotherapy 2
S414 www.painphysicianjournal.com