Page 15 - PC 101 practical notes 24-25..
P. 15
MANSOURA NATIONAL UNIVERSIY
PHARM D- CLINICAL PHARMACY LEVEL I PHARM. ANAL. CHEM. I (PC 101)
Acid-Base Titrations
It's a type of titrations that involve reactions between acids and bases.
Remember: ▪ pH = - log [H ] ……………[H ] means the molar concn. of H .
+ + +
▪ pH > 7 basic, pH =7 neutral, pH < 7 acidic.
Acid-base indicators:
♦ They are indicators used to indicate the end point of acid-base titrations (i.e.
indicate the completeness of the reaction by changing their colors).
♦ Each indicator has its specific {pH range} which is the range of pH within
which the indicator changes its color.
Examples of acid-base indicators:
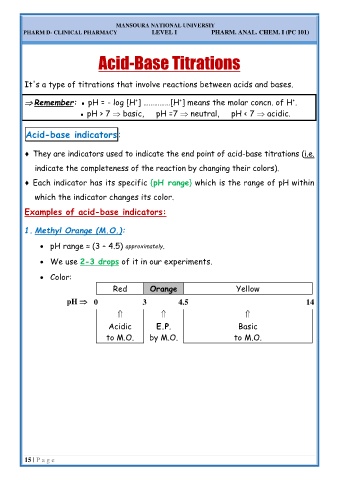
1. Methyl Orange (M.O.):
• pH range ≈ (3 – 4.5) approximately.
• We use 2-3 drops of it in our experiments.
• Color:
Red Orange Yellow
pH 0 3 4.5 14
Acidic E.P. Basic
to M.O. by M.O. to M.O.
15 | P a g e